|
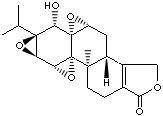
TRIPTOLIDE |
| Triptolid; (3bS,4aS,5aS,6R,6aR,7aS,7bS,8aS,8bS)-3b,4,4a,6,6a,7a,7b,8b,9,10-decahydro-6-hydroxy- 8b-methyl-6a- (1-methylethyl)trisoxireno(4b,5:6,7:8a,9)phenanthro(1,2-c)furan-1(3H)-one; |
|
|
| PRODUCT IDENTIFICATION |
|
|
CAS RN |
38748-32-2 |
|
EINECS RN |
|
|
FORMULA |
C20H24O6 |
|
MOLE WEIGHT |
360.40 |
|
|
| PHYSICAL AND CHEMICAL PROPERTIES |
|
|
PHYSICAL STATE |
white to off-white powder |
|
MELTING POINT |
235 - 237 C |
|
BOILING POINT |
|
|
DENSITY |
|
|
SOLUBILITY IN WATER |
(Soluble in DMSO) |
|
pH |
|
|
VAPOR DENSITY |
|
|
REFRACTIVE INDEX |
|
|
FLASH POINT |
|
|
|
| STABILITY AND REACTIVITY | |
| STABILITY | Stable under normal conditions |
|
INCOMPATIBLE MATERIALS |
Strong acids, Strong bases |
| DECOMPOSITION PRODUCTS |
Carbon oxides. |
| POLYMERIZATION | Has not been reported |
|
NFPA RATINGS |
Health: 1, Flammability: 0, Reactivity: 0 |
|
|
| SAFETY |
|
|
HAZARD NOTES |
|
|
EYE |
May cause eye irritation. |
|
SKIN |
May be harmful if absorbed through skin. May cause skin irritation. |
|
INGESTION |
May be harmful if swallowed. |
|
INHALATION |
May be harmful if inhaled. May cause respiratory tract irritation. |
|
CHRONIC |
|
|
|
| TRANSPORT & REGULATORY INFORMATION |
|
|
UN NO. |
|
| HAZARD CLASS |
|
| PACKING GROUP |
|
| HAZARD CODE |
|
|
RISK STATEMENTS |
|
|
SAFETY STATEMENTS |
22-24/25 |
|
|
| EXTERNAL LINKS & GENERAL INFORMATION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Celastrol has been effectively used in the treatment of autoimmune diseases,
asthma, chronic inflammation, and neurodegenerative disease. Under in vitro
conditions, Celastrol was found to inhibit cancer cell proliferation and induce
leukemic cell death; however, the molecular mechanism involved still remains
unclear. (http://cancerres.aacrjournals.org/)
Tripterine (celastrol) is a pentacyclic triterpenoid antioxidant compound isolated from the Chinese Tripterygium wilfordii Hook. It has an activity of immunosuppressive and anti-inflammatory. Triterpenes antioxidants are not common, but tripterine's antioxidant potency is equivalent to about 15 times that of alpha-tocopherol. Triptolide (PG490, 97% pure) is a diterpenoid triepoxide with potent anti-inflammatory and immunosuppressive effects in transformed human bronchial epithelial cells and T cells (Qiu D, Zhao G, Aoki Y, Shi L, Uyei A, Nazarian S, Ng JC-H, and Kao PN. J Biol Chem 274: 13443-13450, 1999). Triptolide, with an IC50 of ~20-50 ng/ml, inhibits normal and transformed human bronchial epithelial cell expression of interleukin (IL)-6 and IL-8 stimulated by phorbol 12-myristate 13-acetate (PMA), tumor necrosis factor-α, or IL-1β. Nuclear runoff and luciferase reporter gene assays demonstrate that triptolide inhibits IL-8 transcription. Triptolide also inhibits the transcriptional activation, but not the DNA binding, of nuclear factor-κ. A cDNA array and clustering algorithm analysis reveals that triptolide inhibits expression of the PMA-induced genes tumor necrosis factor-α, IL-8, macrophage inflammatory protein-2α, intercellular adhesion molecule-1, integrin β6, vascular endothelial growth factor, granulocyte-macrophage colony-stimulating factor, GATA-3, fra-1, and NF45. Triptolide also inhibits constitutively expressed cell cycle regulators and survival genes cyclins D1, B1, and A1, cdc-25, bcl-x, and c-jun. Thus anti-inflammatory, antiproliferative, and proapoptotic properties of triptolide are associated with inhibition of nuclear factor-κ signaling and inhibition of genes known to regulate cell cycle progression and survival. http://ajplung.physiology.org/cgi/content/full/279/5/L958 ( http://cancerres.aacrjournals.org/)Triptolide, a diterpenoid isolated from the Chinese herb Tripterygium wilfordii Hook F, has been demonstrated to be effective in the treatment of a variety of autoimmune diseases. T helper type 17 (Th17) cells represent a novel subset of CD4(+) T cells involved in the immunopathogenesis of autoimmune diseases. Currently, the effects of triptolide on the differentiation of Th17 cells remain unclear. Here, we found that triptolide significantly inhibited the generation of Th17 cells from murine splenocytes and purified CD4(+) T cells in a dose-dependent manner. The suppressive effects of triptolide were persistent even after it had been removed from cell cultures. (http://www.ncbi.nlm.nih.gov/) Pharmacological actions:
Contraceptive agents
|
|
|
| SALES SPECIFICATION |
|
|
APPEARANCE |
white to yellow crystalline powder |
|
ASSAY |
98.0% min |
|
WATER |
2.0% min |
|
|
| PRICE INFORMATION |
|
|