ANASTROZOLE
PRODUCT IDENTIFICATION
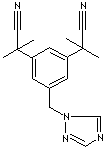
H.S. CODE
TOXICITY
CLASSIFICATION
Aromatase inhibitors
PHYSICAL AND CHEMICAL PROPERTIES
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
Stable under ordinary conditions.
GENERAL DESCRIPTION & APPLICATIONS
Anastrozole is a reversible (Type II), nonsteroidal aromatase inhibitor. Aromatase catalyzes the final and rate-limiting step in the conversion of androgens to estrogens in peripheral tissues. This occurs mainly in adipose tissue, but also in normal and malignant breast tissues, and provides the main source of estrogen in postmenopausal women. The goal of hormone therapy in breast cancer is to deprive tumour cells of estrogens, which are implicated in the development or progression of tumours. Maximal estrogen suppression is produced by a 1mg dose. Estrogen suppression is maintained for up to 6 days after discontinuing anastrozole.6 Differences in the mechanism of action may contribute to the apparent lack of cross-resistance between steroidal (eg, exemestane) and nonsteroidal (eg, anastrozole and letrozole) aromatase inhibitors.7 Highly selective blockade of aromatase does not interfere with the production of other steroids (eg, adrenal corticosteroids, aldosterone) or thyroid stimulating hormone.8 Anastrozole does not have progestogenic, androgenic or estrogenic activity..(http://www.bccancer.bc.ca/)
Aromatase inhibitors lower the amount of estrogen in post-menopausal women who have hormone-receptor-positive breast cancer. The hormone estrogen delivers growth signals to the hormone receptors. With less estrogen in the body, the hormone receptors receive fewer growth signals, and cancer growth can be slowed down or stopped. Before menopause, the ovaries produce most of a woman's estrogen, so reducing estrogen from other sources has little or no effect. But in post-menopausal women, most of the body's estrogen is made from another hormone, androgen. Aromatase inhibitors stop the enzyme called aromatase from turning androgen into estrogen, lowering the amount of estrogen produced OUTSIDE the ovaries. That means less estrogen in the bloodstream, less estrogen reaching estrogen receptors, and less cancer cell growth (http://www.breastcancer.org/)
|
Aromatase Inhibitors |
|
|
Product |
CAS RN. |
|
Aminoglutethimide |
125-84-8 |
| Formestane | 566-48-3 |
| Testolactone | 968-93-4 |
| Plomestane | 77016-85-4 |
| Fadrozole | 102676-47-1 |
| Exemestane | 107868-30-4 |
| Letrozole | 112809-51-5 |
| Vorozole | 118949-22-7 |
| Anastrozole | 120511-73-1 |
Pharmacological actions:
- Antineoplastic agent
- Aromatase Inhibitor
APPEARANCE
ASSAY
0.15% max