PRODUCT IDENTIFICATION
H.S. CODE
TOXICITY
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
SOLVENT SOLUBILITY
REFRACTIVE INDEX
Stable under ordinary conditions
GENERAL DESCRIPTION & EXTERNAL LINKS
|
|
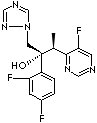
Voriconazole: the newest triazole antifungal agent........INDICATIONS Voriconazole (VFEND, Pfizer Ireland Pharmaceuticals, Ringaskiddy, Ireland) is a triazole antifungal agent that inhibits fungal ergosterol biosynthesis (5). It is structurally related to fluconazole, with the major difference being the substitution of a fluoropyrimidine grouping in place of a triazole moiety (5, 6). Voriconazole is indicated for the treatment of invasive aspergillosis. It is also indicated for the treatment of fungal infections caused by S. apiospermum or Fusarium spp. that are refractory to other antifungal agents (5). PHARMACOLOGY Like the other triazole antifungals, voriconazole exerts its antifungal activity by inhibition of 14-alpha-lanosterol demethylation, which is mediated by fungal cytochrome P450 enzymes (2, 5, 6). This inhibition is more selective for fungal than for mammalian enzyme systems. The accumulation of 14-alphamethyl sterols results in a decrease in ergosterol, which is an essential component of fungal cell wall formation. The resulting cell wall abnormalities are thought to be responsible for voriconazole��s antifungal activity.........(http://www.baylorhealth.edu/)
APPEARANCE
ASSAY
-54° ~ -59°
FLUORIDE CONTENT
14.7 - 17.9%
OPTICAL ISOMER
0.1% max
0.5% max (total), 0.1% max (Individual)
PRICE INFORMATION