PRODUCT IDENTIFICATION
H.S. CODE
TOXICITY
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
Stable under ordinary conditions.
GENERAL DESCRIPTION & APPLICATIONS
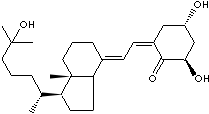
Calcitriol, 1,25-dihydroxycholecalciferol, is the physiologically active form of vitamin D which contributes to the intestinal absorption of Ca2+ and Ca2+ resorption by bone. Calcitriol is a steroid hormone found in the body. Calcitriol is used as a medication in the treatment of hypocalcaemia, hypophosphatemia, rickets, and osteoporosis. Calcifediol (25-hydroxycholecalciferol) is a prehormone which is produced by the metabolism of cholecalciferol. Its production is stimulated by low blood calcium levels and parathyroid hormone. Calcifediol is converted by the kidneys into calcitriol and calcitriol is converted into alfacalcidol in the liver.
| Bone Density Regulators |
CAS RN |
| Alendronate sodium | 121268-17-5 |
| Alendronic acid | 66376-36-1 |
| Alfacalcidol | 41294-56-8 |
| Calcifediol | 63283-36-3 |
| Calcitonin | 47931-85-1 |
| Calcitriol | 32222-06-3 |
|
Cholecalciferol |
67-97-0 |
| Clodronic acid | 10596-23-3 |
| Dihydrotachysterol | 67-96-9 |
| Disodium cycloheptylaminomethylenediphosphonate | 138330-18-4 |
| Ergocalciferol | 50-14-6 |
| Etidronate Disodium | 7414-83-7 |
| Etidronic acid | 2809-21-4 |
| Gallium nitrate | 135886-70-3 |
| Hydroxycholecalciferols |
|
| Ibandronate sodium hydrate | 138926-19-9 |
|
Ibandronic acid |
114084-78-5 |
|
Olpadronic acid |
63132-39-8 |
| Oxidronic acid | 15468-10-7 |
| Pamidronate disodium | 109552-15-0 |
| Pamidronic acid disodium salt | 57248-88-1 |
| Pamidronic acid | 40391-99-9 |
| Potassium phosphate dibasic | 7758-11-4 |
| Procalcitonin | 56645-65-9 |
|
Raloxifene |
84449-90-1 |
| Risedronate sodium | 115436-72-1 |
|
Risedronic acid |
105462-24-6 |
| Secalciferol | 55721-11-4 |
|
Tamoxifen |
10540-29-1 |
|
Teriparatide |
52232-67-4 |
|
Tiludronic acid |
89987-06-4 |
| Toremifene citrate | 89778-27-8 |
|
Toremifene |
89778-26-7 |
|
Vitamin D |
1406-16-2 |
| Vitamin D2 sulfate | 1784-46-9 |
|
Zoledronic acid |
118072-93-8 |
| 24,25-Dihydroxyvitamin D 3 | 40013-87-4 |
|
25-Hydroxyvitamin D 2 |
21343-40-8 |
APPEARANCE
ASSAY
1alpha, 25-Dihydroxy-trans-vitamin D3: 0.5% max
Pre-calcitriol: 0.5% max
Individual unspecified substances: 0.1% max
Total related substances: 1.0% max
Acetone: 5000ppm max
Methanol: 0.3% max
Other residual solvent: 0.1% max