|
Propionic
acid is a clear, colorless liquid with a slightly sweetish odor. It is
soluble in water and alcohol; melts at -21 C, boils at 141 C. It is used as a
preservative in feed and food industry directly or in the forms of ammonium
propionate, calcium and sodium propionates. It is used to manufacture various
propionates which used in the reduction of pharmaceuticals, anti-fungal agents,
agrochemicals, plastics, plasticizers, rubber chemicals, dyes, artificial
flavors and perfumery synthetics. It is used also as a solvent and in
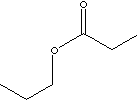
nickel-electroplating solutions. Propyl Propionate
is used in
perfumery and fragrance.
Odor description: sweet, fruity, pineapple,
apple, banana, bilberry, cider, cranberry, durian. |
|
Solvent is a
substance, usually a liquid, that acts as a dissolving agent or that is capable
of dissolving another substance. In solutions of solids or gases in a liquid,
the liquid is the solvent. In all other homogeneous mixtures (i.e., liquids,
solids, or gases dissolved in liquids; solids in solids; and gases in gases.),
solvent is the component of the greatest amount. The minor proportion substances
are called solutes. The solvent offers several functions during a chemical
reaction. It solves the substance that reacts with another one to produce a new
set of substances (reactant) and the compound that supplies the molecule, ion,
or free radical which is considered as the attacking species in a chemical
reaction (reagent). The solvent is conductive to collisions between the
reactants and reagents to transform the reactants to new products. The solvent
also takes roll of temperature control, either to provide the energy of the
colliding particles for speedy reaction and to absorb heat in exothermic
reaction. The appropriate solvent should be selected based on the inactivity in
the reaction conditions, dissolving the reagents as well as reactants,
appropriate boiling point and easy removal at the end of the reaction. he most
common solvent is water. Other common solvents which dissolve substances that
are insoluble (or nearly insoluble) in water are acetone, alcohol, formic acid,
acetic acid, formamide. BTX, carbon disulfide, diemthyl sulfoxide, carbon
tetrachloride, chloroform, ether, tetrahydrofuran, furfural, hexane and
turpentine. They may be classified as polar and nonpolar types. They may be
classified as polar and nonpolar types. Polar solvents, like water, have
molecules whose electric charges are unequally distributed, leaving one end of
each molecule more positive than the other. Usually polar solvent has O-H bond
of which water (HOH), methanol (CH3OH) and acetic acid
(CH3COOH) are examples.
Propanol, butanol, formic acid, formamide are polar solvents. Dipolar solvents
which contain a C-O solid bond without O-H bond are acetone
[(CH3)2C=O],
ethyl acetate (CH3COOCH2CH3), methyl ethyl
ketone, acetonitrile, N,N-dimethylformamide and diemthyl sulfoxide. Nonpolar
solvents, like carbon tetrachloride (CCl4), benzene
(C6H6), and diethyl ether (
CH3CH2OCH2CH3), have molecules
whose electric charges are equally distributed and are not miscible with water.
Hexane, tetrahydrofuran and methylene chloride are nonpolar
solvents. |