VALERIC ACID
PRODUCT IDENTIFICATION
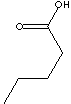
102.13
H.S. CODE
2915.60.9000
TOXICITY
SMILES
CLASSIFICATION
Straight chain fatty acid, Flavors & Fragrances, Biochemical ex Plants
PHYSICAL AND CHEMICAL PROPERTIES
0.934-0.939
NFPA RATINGS
REFRACTIVE INDEX
1.405-1.414
95 C
GENERAL DESCRIPTION & EXTERNAL LINKS
Valeric
acid ( pentanoic acid or propylacetic
acid in systemic naming) is a member of short
chain straight
fatty acids. It is a colorless liquid with
a penetrating aroma; slightly
soluble
in water, soluble in alcohol, and ether.
It melts at -34 C and boils at 186 C . ;
boils at 185 C
. Isovaleric acid (3-methylbutanoic acid ) is a member of branched fatty acids. It is a colorless liquid;
slightly
soluble
in water, soluble in alcohol, and almost organic solvents
including ethers. It has a strong pungent sweaty smell. It melts
at -29 C and boils at 176 C. Their
primary application
is in the synthesis of its esters which are more
volatile
than their parent compounds. Valeric
esters have distinctive fruit-like odors,
which has led to the use in
fruity flavors, perfume
and cosmetics. (e.g: Methyl valerate:flowery,
Ethyl valerate: fruity particularly apple, Ethyl
isovalerate:apple, Amyl valerate: apple and pineapple).
Ester smells:http://www.chm.bris.ac.uk/
There are almost infinite esters obtained from thousands of potential starting materials. Esters are formed by removal of water from an acid and an alcohol, e.g., carboxylic acid esters, phosphoric acid esters, and sulfonic acid esters. Carboxylic acid esters are used as in a variety of direct and indirect applications. Lower chain esters are used as flavouring base materials, plasticizers, solvent carriers and coupling agents. Higher chain compounds are used as components in metalworking fluids, surfactants, lubricants, detergents, oiling agents, emulsifiers, wetting agents textile treatments and emollients, They are also used as intermediates for the manufacture of a variety of target compounds. The almost infinite esters provide a wide range of viscosity, specific gravity, vapor pressure, boiling point, and other physical and chemical properties for the proper application selections.
Valeric acid, isovaleric acid their esters are useful raw material for variety of industrial target compounds including;
- Plasticizers and Lubricants
- Biodegradable solvents and lubricants
- Engineering plastics
- Epoxy curing agents
- Adhesive and powder coatings
- Corrosion inhibitors
- Perfumery and pharmaceuticals
- Electrolytes
- Vinyl stabilizers
Esters in lubrication: http://www.forearthonline.com/
Carboxylic Acids: http://www2.chemistry.msu.edu/Wikipedia Linking:http://en.wikipedia.org/wiki/Valeric_acid, http://en.wikipedia.org/wiki/Isovaleric_acid
SALES SPECIFICATION
APPEARANCE
CONTENT
98.0% min
ACID VALUE
538 - 550 ( mg KOH/g)
MOISTURE
0.5% max
8
|
ALIPHATIC CARBOXYLIC ACIDS |
||||
|
COMMON NAME | SYSTEMATIC NAME |
CAS RN | FORMULA |
MELTING POINT |
| Formic Acid | Methanoic acid | 64-18-6 | HCOOH | 8.5 C |
| Acetic Acid | Ethanoic acid | 64-19-7 | CH3COOH |
16.5 C |
| Carboxyethane | Propionic Acid | 79-09-4 | CH3CH2COOH |
-21.5 C |
| Butyric Acid | n-Butanoic acid | 107-92-6 | CH3(CH2)2COOH |
-8 C |
| Valeric Acid | n-Pentanoic Acid | 109-52-4 | CH3(CH2)3COOH |
-19 C |
| Caproic Acid | n-Hexanoic Acid | 142-62-1 | CH3(CH2)4COOH |
-3 C |
| Enanthoic Acid | n-Heptanoic acid | 111-14-8 | CH3(CH2)5COOH |
-10.5 C |
| Caprylic Acid | n-Octanoic Acid | 124-07-2 | CH3(CH2)6COOH |
16 C |
| alpha-Ethylcaproic Acid | 2-Ethylhexanoic Acid | 149-57-5 | CH3(CH2)3CH(C2H5)COOH |
-59 C |
| Valproic Acid | 2-Propylpentanoic Acid | 99-66-1 | (CH3CH2CH2)2CHCOOH |
120 C |
| Pelargonic Acid | n-Nonanoic Acid | 112-05-0 | CH3(CH2)7COOH |
48 C |
| Capric Acid | n-Decanoic Acid | 334-48-5 | CH3(CH2)8COOH |
31 C |