| CAS
NO. |
137-26-8 |
|
| EINECS NO. |
205-286-2 |
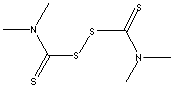
| FORMULA |
[(CH3)2NCS2-]2 |
| MOL
WT. |
240.43 |
|
H.S.
CODE
|
2930.30 |
|
TOXICITY
|
Oral, rat LD50: 560 mg/kg |
| SYNONYMS |
Thiram; Bis(dimethylthiocarbamoyl)disulfide;
|
| TMTD; Tetramethylthioperoxydicarbonothioic
diamine; Thirame (French); Bis(Dimethyl-thiocarbamoyl)-Disulfid (German); Bis(Dimethylthiocarbamoyl)
Disulfide; Bis(Dimethylthiocarbamoyl) Disulphide; Bis(Dimethylthiocarbamyl) Disulfide;
Disolfuro Di Tetrametiltiourame (Italian);
Disulfure De
Tetramethyl Thiourame (French); Alpha,Alpha'-dithiobis(Dimethylthio)
Formamide; N,N'-(Dithiodicarbonothioyl) Bis(N-methylmethanamine); Tetramethyldiurane Sulphite;
Tetramethylenethiuram Disulphide;
Tetramethylthiocarbamoyl Disulphide; Tetramethylthioperoxydicarbonic
Diamide; Tetramethyl Thioramdisulfide (Dutch); Tetramethyl-thiram
Disulfid (German); Tetramethylthiuram Bisulfide; Tetramethylthiuram
Bisulphide; Tetramethylthiuram Disulfide; Tetramethylthiuram
Disulphide; N,N-tetramethylthiuram Disulphide; N,N,N',N'-
Tetramethylthiuram Disulfide; Tetramethylthiuran Disulphide;
Tetramethyl Thiurane Disulfide; Tiuram
(Polish); |
| SMILES |
|
|
CLASSIFICATION
|
DISINFECTANTS
/
RUBBER ACCELERATORS
/
|
|
PHYSICAL AND CHEMICAL PROPERTIES
|
| PHYSICAL
STATE |
white crystalline powder |
|
MELTING
POINT
|
155
C |
| BOILING
POINT |
129
C |
| SPECIFIC GRAVITY |
1.425 |
|
SOLUBILITY
IN WATER
|
Insoluble
|
| pH |
|
| VAPOR DENSITY |
|
|
AUTOIGNITION |
|
|
REFRACTIVE
INDEX
|
|
| NFPA RATINGS |
Health:
2, Flammability: 1, Reactivity: 0
|
| FLASH
POINT |
138
C
|
| STABILITY |
Stable
under ordinary conditions |
|
APPLICATIONS
|
|
This compound is used as a fungicide, seed disinfectant, bactericide,
animal repellent,
insecticide. It is used as a primary and secondary accelerator or as a sulfur
donor (vulcanizing agent) in most sulfur-cured elastomers in rubber industry. It is also used
in the treatments of chronic
alcoholism
with cause of acetaldehyde, a breakdown product of alcohol, to accumulate in the blood. It
is a peptizing agent in sulfur-modified polychloroprenes. It is used in soaps and rodent repellents
and as a nut, fruit and mushroom disinfectant. |
| SALES
SPECIFICATION |
|
APPEARANCE
|
white
to off white
powder
|
|
ASSAY
|
97.0%
min
|
|
MELTING
POINT
|
142
C (Initial), 150 - 157 C (Final)
|
|
SIEVE
ANALYSIS
|
0.1% max (+ 150 µm)
|
|
HEAT
LOSS
|
0.3%
max
|
|
OIL
ADDITIVE
|
1.0
- 2.0%
|
|
ASH
|
0.3%
max
|
TRANSPORTATION |
| PACKING |
25kgs
in Bag |
| HAZARD CLASS |
|
| UN
NO. |
2771 |
| GENERAL
DESCRIPTION OF ACCELERATOR |
|
Sulfur combines with nearly all elements. Sulfur forms ring and chain structures
as it is the second only to carbon in exhibiting catenation. The 8-membered ring and
shorter chain structure of sulfur molecule is important in vulcanization
process which individual polymers are linked to other polymer molecules by
atomic bridges. This process produces thermoset materials which are cross-linked
and irreversible substances. The term thermoplastic is for high molecular weight
polymers which can undergo melting-freezing cycle. Thermosets are not melted and
re-molded on heating after cured. The split of sulfur 8-membered ring structure into shorter chains provides rubber vulcanization process. The split are
liked with cure sites (some of the solid bonds in the molecule) on rubber
molecules, resulting in forming sulfur bridges typically between 2 and 10 atoms
long. Vulcanization makes rubber harder, more durable and more resistant to
heating, aging and chemical attacks. The number of sulfur atoms in the sulfur bridges varies physical properties of
the end products. Short bridges containing one or two sulfur atoms offer heat
resistance and long bridges offer flexible property. Vulcanization can also be accomplished with
certain peroxides, gamma radiation, and several other organic compounds. The
principal classes of peroxide cross-linking agents are dialkyl and diaralkyl
peroxides, peroxyketals and peroxyesters. Other vulcanizing agents include amine
compounds for the cross-linking of fluorocarbon rubbers, metal oxides for
chlorine-containing rubbers (notably zinc oxide for chloroprene rubber) and
phenol-formaldehyde resins for the production of heat-resistant butyl rubber
vulcanizates. Accelerator, in the rubber industry, is added with a curing agent
to speed the vulcanization. Accelerators contain sulfur and nitrogen like derivatives of benzothiazole and thiocarbanilides.
The popular accelerators are
sulfenamides (as a delayed-action accelerators), thiazoles, thiuram sulfides,
dithocarbamates and guanidines.
There are some types of rubber accelerators. They are used in combination with each other in
accordance with vulcanizing and/or acid-base conditions. Some examples
classified by chemical structure are as below;
- Thiazole
- 2-Mercaptobenzothiazole (CAS #:
149-30-4)
- Dibenzothiazole disulfide (CAS #:
120-78-5)
- 2-Mercaptobenzothiazole Zinc salt (CAS #:
155-04-4)
- Sulphenamide
- N-Cyclohexyl-2-benzothiazole sulfenamide (CAS #:
95-33-0)
- N-Oxydienthylene-2-benzothiazole sulfenamide (CAS #:
102-77-2)
- N-tert-butyl-2-benzothiazyl sulfenamide (CAS #:
95-31-8)
- Guanidine
- Diphenyl
guanidine (CAS #: 102-06-7)
- Di-o-tolylguanidine (CAS #: 97-39-2)
- Thiuram
- Tetramethyl
thiuram disulfide (CAS #: 137-26-8)
- Tetraethyl
thiuram disulfide (CAS #: 97-77-8)
- Tetramethyl
thiuram monosulfide (CAS #: 97-74-5)
- Isobutyl
thiuram disulfide (CAS #: 3064-73-1)
- Tetrabenzylthiuram disulfide (CAS #:
10591-85-2)
- Dipentamethylene thiuramtetrasulfide (CAS #:
120-54-7)
- Dithiocarbamate
- Zinc
dimethyl dithiocarbamate (CAS #: 137-30-4)
- Zinc diethyl
dithiocarbamate (CAS #: 14324-55-1)
- Zinc dibutyl
dithiocarbamate (CAS #: 136-23-2)
- Zinc
N-ethyl-dithiocarbamate (CAS #: 14634-93-6)
- Zinc
dibenzyl dithiocarbamate (CAS #: 14726-36-4)
- Copper
dimethyl dithiocarbamate (CAS #: 137-29-1)
- Thiourea
- Ethylene
thiourea (CAS #: 96-45-7)
- N,N'-Diethylthiourea (CAS #: 105-55-5)
- N-N'-Diphenylthiourea (CAS #:
102-08-9)
|
|
PRICE
|
|

|
