PRENOL
PRODUCT IDENTIFICATION
556-82-1
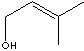
H.S. CODE
TOXICITY
Dimethylallyl alcohol; 3,3-Dimethylallyl alcohol; 3-Methyl-2-butenol; 3-Methyl-2-butenyl alcohol; 3-Methylcrotyl alcohol; gamma,gamma-Dimethylallyl Alcohol;
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
colorless liquid with fruity odor
MELTING POINT
SOLUBILITY
Reasonably soluble in water; soluble in alcohol
REFRACTIVE INDEX
Stable under ordinary conditions
GENERAL DESCRIPTION & EXTERNAL LINKS
Wikipedia linking: http://en.wikipedia.org/wiki/Prenol
Prenols (PR) are synthesized from the five carbon precursors isopentenyl diphosphate and dimethylallyl diphosphate that are produced mainly via the mevalonic acid pathway In some bacteria (e.g., Escherichia coli) and plants, isoprenoid precursors are made by the methylerythritol phosphate pathway. Because the simple isoprenoids(linear alcohols, diphosphates, etc.) are formed by the successive addition of C5 units, it is convenient to classify them in this manner, with a polyterpene subclass for those structures containing more than 40 carbons(i.e., 8 isoprenoid units). Note that vitamin A and its derivatives and phytanic acid and its oxidation product pristanic acid are grouped under C20 isoprenoids. Carotenoids are important simple isoprenoids that function as antioxidants and as precursors of vitamin A. Another biologically important class of molecules is exemplified by the quinones and hydroquinones, which contain an isoprenoid tail attached to a quinonoid core of nonisoprenoid origin. Vitamins E and K as well as the ubiquinones are examples of this class. PR and their phosphorylated derivatives play important roles in the transport of oligosaccharides across membranes. Polyprenol phosphate sugars and polyprenol diphosphate sugars function in extracytoplasmic glycosylation reactions, in extracellular polysaccharide biosynthesis [for instance, peptidoglycan polymerization in bacteria], and in eukaryotic protein N-glycosylation. The biosynthesis and function of polyprenol phosphate sugars differ significantly from those of the polyprenol diphosphate sugars. Bacteria synthesize polyprenols(called bactoprenols) in which the terminal isoprenoid unit attached to oxygen remains unsaturated, whereas in animal polyprenols (dolichols) the terminal isoprenoid is reduced. Bacterial polyprenols are typically 10 to 12 units long, whereas dolichols usually consist of 18 to 22 isoprene units. In the phytoprenols of plants, the three distal units are reduced. (http://www.lipidomicnet.org/)
Lipids may be broadly defined as hydrophobic or amphiphilic small molecules; the amphiphilic nature of some lipids allows them to form structures such as vesicles, liposomes, or membranes in an aqueous environment. Biological lipids originate entirely or in part from two distinct types of biochemical subunits or "building blocks": ketoacyl and isoprene groups. Using this approach, lipids may be divided into eight categories: fatty acyls, glycerolipids, glycerophospholipids, sphingolipids, saccharolipids and polyketides (derived from condensation of ketoacyl subunits); and sterol lipids and prenol lipids (derived from condensation of isoprene subunits). Although the term lipid is sometimes used as a synonym for fats, fats are a subgroup of lipids called triglycerides. Lipids also encompass molecules such as fatty acids and their derivatives (including tri-, di-, and monoglycerides and phospholipids), as well as other sterol-containing metabolites such as cholesterol. Although humans and other mammals use various biosynthetic pathways to both break down and synthesize lipids, some essential lipids cannot be made this way and must be obtained from the diet (http://www.news-medical.net/)
APPEARANCE
colorless liquid with fruity odor
98.0% min