| CAS
NO. |
473-55-2
|
|
| EINECS
NO. |
229-978-9 |
| FORMULA |
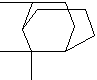
C10H18 |
| MOL
WT. |
138.25 |
| H.S.
CODE |
2902.19 |
| TOXICITY |
|
| SYNONYMS |
2,6,6-trimethyl-,
(1a,2b,5a)-Bicyclo[3.1.1]Heptane; |
| Dihydropinene;
2,7,7-trimethylbicyclo[3.1.1]heptane; |
| SMILES |
hydrogenation
of alpha-pinene
|
|
CLASSIFICATION
|
|
|
PHYSICAL
AND CHEMICAL PROPERTIES
|
| PHYSICAL
STATE |
Colorless
to pale yellow liquid
|
| MELTING
POINT |
|
| BOILING
POINT |
168
- 169 C |
| SPECIFIC
GRAVITY |
0.857
|
| SOLUBILITY
IN WATER |
|
|
SOLVENT
SOLUBILITY
|
|
| pH |
|
| VAPOR
DENSITY |
|
|
AUTOIGNITION
|
|
|
NFPA
RATINGS
|
Health:
1 Flammability: 1 Reactivity: 1 |
|
REFRACTIVE
INDEX
|
1.4622
|
| FLASH
POINT |
|
| STABILITY |
Stable
under ordinary conditions |
|
GENERAL
DESCRIPTION & APPLICATIONS
|
|
Turpentine is a
semifluid substance consisting of two principal components, spirits of
turpentine (volatile portion also known as oil of turpentine or turps ) and a
type of rosin (nonvolatile portion also known as colophony). Turpentine is
exuded from coniferous trees. The crude turpentine is distilled through steam
into commercial turpentine, oil of turpentine. The pure turpentine oil is a
transparent oily liquid with a penetrating odor and a characteristic taste.
Turpentine is not a pure substance but a complex mixture of terpenes,
particularly large proportion of pinene (bicyclic monoterpenic hydrocarbon), a
compound from which camphor is manufactured. Terpene is a class of naturally
occurring unsaturated hydrocarbons whose carbon skeletons are composed
exclusively of isoprene C5 units (CH2=C(CH3)-CH=CH2), consisting of five carbon atoms attached to eight
hydrogen atoms (C5H8). The stepwise
distillation with water and carbonates yields terpenes. The most abundant monoterpene
is pinene.
There are two isomerers called alpha (or
beta) pinene. The alpha form boils at 156-160 C, and of the beta form boils at
164-169C. It is used as solvents for paints, coatings and wax formulations. It
is used as an intermediate for resins and for camphor, menthol, campholenic aldehyde
and terpineol.
It is used as lube-oil additives. Myrcene,one
of the most important chemicals used in the perfumery industry,
is
obtained from beta-Pinene
through
pyrolysis.
Pinane,
hydrogenated alpha-pinene,
is used as an intermediate for the production
of terpene alcohols and other fragrance chemicals.
|
| SALES
SPECIFICATION |
|
APPEARANCE
|
Colorless
to pale yellow liquid
|
|
cis-PINANE
|
95.0%
min
|
|
trans-PINANE
|
5.0%
max
|
|
BOILING
POINT
|
167
C min |
| TRANSPORTATION |
| PACKING |
175kgs
in drum |
| HAZARD
CLASS |
3
(Packing group: III) |
| UN
NO. |
2319 |
| GENERAL
DESCRIPTION OF TERPENE
|
A class of naturally occurring compounds mainly in
plants as constituents of essential oils whose carbon skeletons are composed
exclusively of isoprene C5 units (CH2=C(CH3)-CH=CH2). Most terpenes are
hydrocarbons having molecular formula (C5H8)n in a cyclic or
acyclic, saturated or unsaturated structure, while the terpenoids are
oxygen-containing analogues of the terpenes such as alcohols, aldehydes or
ketones containing hydroxyl groups or carbonyl groups. Several vitamines,
hormones, flavour and flagrances and latex are terpenoids. Terpenes containing
30 or more carbons are usually formed by the fusion of two terpene precursors in
a regular pattern, usually head-to-tail appears to be violated. They are differ
from one another not only in functional groups but also in their basic carbon
skeletons. Terpenes are employed mainly the fragrance and flavour purpose, as
well as in the pharmaceutical and chemical industries. They are classified by
the number of isoprene units:
|
Class |
number of
isoprene |
Examples |
|
Hemiterpene |
1
(C5H8) |
Found
in associated with Alkaloids, Coumarins and Flavonoids. |
|
Monoterpenes |
2
(C10H16) |
Geraniol, Citronellol, Pinene, Nerol, Citral,
Camphor, Menthol, Limonene, Thujone |
|
Sesquiterpenes |
3
(C15H24) |
Nerolidol, Farnesol |
|
Diterpenes |
4
(C20H32) |
Phytol, Vitamin A1 |
|
Triterpenes |
6
(C30H48) |
Squalene |
|
Tetraterpenes |
8
(C40H84) |
Carotene (Provitamin A1) |
|
Polyterpenes |
>10
(C5H8)n |
| |
