| CAS
NO. |
1072-83-9 |
|
| EINECS
NO. |
214-016-2 |
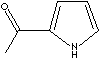
| FORMULA |
C6H7NO |
| MOL
WT. |
109.13 |
|
H.S.
CODE
|
2933.90 |
|
TOXICITY
|
|
| SYNONYMS |
2-Acetylpyrrole;
2-Pyrrolyl methyl ketone; |
| 1-(1H-pyrrol-2-yl)-Ethanone;
Methyl pyrrol-2-yl ketone; 2-Acetyl-1H-pyrrole; Methyl pyrrol-2-yl
Ketone; |
| SMILES |
|
|
CLASSIFICATION
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES
|
| PHYSICAL
STATE |
white
to brown crystals |
|
MELTING
POINT
|
86
- 92 C |
| BOILING
POINT |
|
| SPECIFIC GRAVITY |
|
|
SOLUBILITY
IN WATER
|
Soluble
in hot water (Soluble in alcohol) |
| pH |
|
| VAPOR DENSITY |
|
| AUTOIGNITION |
|
|
REFRACTIVE
INDEX
|
|
| NFPA RATINGS |
Health: 1; Flammability: 1; Reactivity: 0 |
| FLASH
POINT |
|
| STABILITY |
Stable under ordinary
conditions |
|
APPLICATIONS
|
|
Methyl-2-Pyrrolyl Ketone
is used as an ingredient in fragrance.
It's end applications include roasted food
and tobaco. (Oral description : nutty, sweet, herbal.) |
| SALES
SPECIFICATION |
|
APPEARANCE
|
white
to brown crystals |
| PURITY |
98.5%
min (G.C) |
|
MELTING
POINT
|
86
- 92 C |
| TRANSPORTATION |
| PACKING |
25kgs
in drum |
| HAZARD CLASS |
|
| UN
NO. |
|
| OTHER
INFORMATION |
FEMA
No.: 3202
Hazard
Symbols: n/a, Risk Phrases: 36/37/38, Safety Phrases: 24/25-28A-37-45 |
|
GENERAL DESCRIPTION PYRROLE |
|
Pyrrole: One of a class of organic heterocyclic compounds of five-membered
diunsaturated ring structure composed of four carbon atoms and one nitrogen
atom. The simplest member of the pyrrole family is pyrrole itself, a basic
heterocyclic compound; colorless to pale yellow, toxic oil with pungent taste
and similar to chloroform odor; insoluble in water; soluble in alcohol, ether,
and dilute acids; boils at 129 - 131 C; polymerizes in light. Pyrrole ring
system is involved in coloured products (green pigment, chlorophyll; red,
hemoglobin; , blue, indigo) in nature. Pyrrolidine, the saturated
tetrahydropyrrole, is part of the structures of amino acids (proline,
hydroxyproline and hygrine). Pyrroline is a pyrrole in which one of the two
solid bonds has been hydrogenated. Pyrrole and its derivatives are widely used
as an intermediate in synthesis of pharmaceuticals, medicines,
agrochemicals, dyes, photographic chemicals, perfumes and other organic
compounds. They are also used as catalysts for polymerization process, corrosion inhibitors,
preservatives, and as solvents for resins and terpenes. They are used in
metallurgical processes. They are useful in the intensive study of transition-metal complex catalyst chemistry for uniform polymerization,
luminescence chemistry and spectrophotometric analysis.
|
