PRODUCT IDENTIFICATION
H.S. CODE
TOXICITY
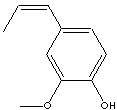
Isoeugenol; 1-(3-Methoxy-4-hydroxyphenyl)-1-propene; 1-Hydroxy-2-methoxy-4-propen-1-ylbenzene; 1-Hydroxy-2-methoxy-4-propenylbenzene; 2-Methoxy-4-(1-propenyl)phenol; 2-Methoxy-4-propenylphenol; 3-Methoxy-4-hydroxy-1-propen-1-ylbenzene; 4-Hydroxy-3-methoxy-1-propen-1-ylbenzene; 4-Hydroxy-3-methoxy-1-propenylbenzene; 4-Hydroxy-3-methoxypropenylbenzene;
CLASSIFICATION
EXTRA NOTES
FEMA No. 2468
PHYSICAL AND CHEMICAL PROPERTIES
MELTING POINT
SOLUBILITY IN WATER
SOLVENT SOLUBILITY
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
112 C
EXTERNAL LINKS & GENERAL DESCRIPTION
Drug Information Portal (U.S. National Library of Medicine) - Isoeugenol
PubChem Compound Summary - Isoeugenol
IPCS INCHEM - Isoeugenol
KEGG (Kyoto Encyclopedia of Genes and Genomes) - Isoeugenol
http://www.ebi.ac.uk/chebi/ - Isoeugenol
http://www.ncbi.nlm.nih.gov/ - Isoeugenol
Human Metabolome Database - Isoeugenol
http://www.pnas.org/
Phenylpropenes
such as chavicol, t-anol, eugenol, and isoeugenol are produced by
plants as defense compounds against animals and microorganisms and
as floral attractants of pollinators. Moreover, humans have used
phenylpropenes since antiquity for food preservation and flavoring
and as medicinal agents. Previous research suggested that the phenylpropenes
are synthesized in plants from substituted phenylpropenols, although
the identity of the enzymes and the nature of the reaction mechanism
involved in this transformation have remained obscure. We show here
that glandular trichomes of sweet basil (Ocimum basilicum), which
synthesize and accumulate phenylpropenes, possess an enzyme that
can use coniferyl acetate and NADPH to form eugenol. Petunia (Petunia
hybrida cv. Mitchell) flowers, which emit large amounts of isoeugenol,
possess an enzyme homologous to the basil eugenol-forming enzyme
that also uses coniferyl acetate and NADPH as substrates but catalyzes
the formation of isoeugenol. The basil and petunia phenylpropene-forming
enzymes belong to a structural family of NADPH-dependent reductases
that also includes pinoresinol–lariciresinol reductase, isoflavone
reductase, and phenylcoumaran benzylic ether reductase.
http://www.scribd.com/
Vanillin
is undoubtedly one of the most popular and widely used flavoring
agents in the world. Naturalvanillin is extracted from vanilla beans
and is relatively expensive. Moreover, the consumer demand for natural
vanillin highly exceeds the amount of vanillin extracted by plant
sources. This had led to theinvestigation of alternative routes
for its production such as microbial bioconversion. In this study,
a novelstrain bacterium capable of converting isoeugenol to vanillin
was isolated by conventional enrichment process from soils of spicy
plants farms. On the basis of morphological and physiochemical characteristics,the
isolate was identified asPseudomonassp. strain ISPC2. Vanillin formation
was analyzed byspectrophotometery with thiobarbituric acid reagent
and evaluated accurately by gas chromatography. Using10 g/l of isoeugenol
as substrate in 25 ml reaction solution at 30 °C and 150 rpm,
vanillin reached amaximum concentration of 1.15 g/l after 96 h reaction,
corresponding to a molar yield of 12.4%. This strainshowed potential
to be a good candidate for biotechnological production of vanillin
from isoeugenol. Further studies for standardization and optimization
for higher yield of vanillin production needs to be investigated
Local: Eugenol, allyl chain-substituted guaiacol (2-methoxyphenol), is a clear to pale yellow oily liquid extracted from certain essential oils especially from clove oil and cinnamon. It is very slightly soluble in water and soluble in organic solvents. It has a spicy odor and taste of clove. Eugenol is used in perfumeries, flavorings, essential oils and in medicine (local antiseptic and analgesic). It is used in the production of isoeugenol for the manufacture of vanillin. Eugenol has wide application in dentistry for analgesic and antiseptic properties. Eugenol derivatives or methoxyphenol derivatives in wider classification are used in perfumery and flavoring. They are used in formulating insect attractants and UV absorbers, analgesics, biocides and antiseptics. They are also used in manufacturing stabilizers and antioxidants for plastics and rubbers. Isoeugenol is used in manufacturing perfumeries, flavorings, essential oils (odor description: Clove, spicy, sweet, woody) and in medicine (local antiseptic and analgesic) as well as vanillin.
APPEARANCE
ASSAY
99.0% min
HAZARD OVERVIEW
OSHA Hazards:Harmful by ingestion., Skin sensitiser, Irritant. May cause respiratory tract irritation. May be harmful if swallowed. Causes eye and skin irritation.
GHS
PICTOGRAMS

HAZARD STATEMENTS
H302
Harmful if swallowed.
H315 Causes skin irritation.
H317 May
cause an allergic skin reaction.
H319 Causes serious eye irritation.
H335
May cause respiratory irritation.
PRECAUTIONARY STATEMENTS
P261
Avoid breathing dust/ fume/ gas/ mist/ vapours/ spray.
P280 Wear
protective gloves.
P305 + P351 + P338 IF IN EYES: Rinse cautiously
with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing.
![]() Xn
Harmful
Xn
Harmful
RISK PHRASES
22
Harmful if swallowed
36/37/38
Irritating to eyes, respiratory system and skin
43
May cause sensitization by skin contact
SAFETY PHRASES
26
In case of contact with eyes, rinse immediately with
plenty of water and seek medical advice
36/37
Wear suitable protective clothing and gloves
PRICE INFORMATION