|
The carboxyl group
(–COOH) can react with alcohols,
forming numerous esters. Many esters have distinctive odors, which are commonly used as fragrance or flavour agents.
Salicylates has a pleasant odor resembling wintergreen.
Salicylates also have analgesic,
antipyretic,
and antiinflammatory
activity.
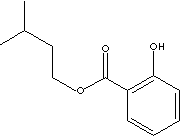
Isoamyl Salicylate
is used in
perfumery and fragrance.
Odor description: herbal woody orchid floral.
|
Salicylic Acid is a white crystalline powder or needle-shaped crystals with
sweetish taste; soluble in acetone, ether, alcohol, boiling water, benzene and
turpentine, sparingly soluble in chloroformbenzene, slightly soluble in water;
melts at 158°C. The sodium salt form (sodium salicylate) is common commercially,
prepared from mainly sodium phenolate with carbon dioxide under heating and
pressure. It contains both a hydroxyl and a carboxyl group, which react with
either an acid or an alcohol. The carboxyl group forms esters with alcohols;
e.g. methyl salicylate is formed with methanol, which used in food flavorings
and preservatives; menthyl salicylate is formed with methanol, which is used in
suntan lotions. The hydroxyl group reacts with acetic acid to form
acetylsalicylic acid (called aspirin) which is the most widely common antiseptic
and antipyretic agent. Phenyl salicylate (called salol) is formed with phenol,
which is also used as an antiseptic and antipyretic agent. The sodium salt
(Sodium salicylate), a shiny white powder, is used for antiseptics preparations
and as a preservative. In addition to its analgesic and antipyretic properties,
salicylic acid possesses keratinolytic properties and fungicidal properties. It
ans its derivatives are used in the treatment of hyperkeratotic, dandruff,
ichthyosis and psoriasis as well as in the treatment of fungal skin infections
such as tinea. Para-Aminosalicylic acid (abbreviated PAS and PASA) is an
analogue of para-aminobenzoic acid (abbreviated PABA) that inhibits folic acid
synthesis in Mycobacterium tuberculosis and is bacteriostatic, inhibits growth
and multiplication of the tubercle bacillus. Para-Aminosalicylic acid and its
sodium salt (sodium p-Aminosalicylate) are bacteriostatic against mycobacteria
and used in the treatment of tuberculosis; administered orally. Brand names are
Tubasal, Nemasol Sodium and etc. Aminosalicylic acids are pharmaceutically
active ingredients including anti-infectives against colds, flu, or other virus
infections. Mesalamine (5-aminosalicylic acid, abbreviated 5-ASA) an active
metabolite of sulfasalazine, used to treat inflammation of the rectum and lower
colon, mild to moderate ulcerative colitis proctosigmoiditis, and proctitis. Para-Aminosalicylic acid
(4-hydroxybenzoic
acid) is used as an intermediate of bacteriostatic agent
specially for parabens (alkyl esters of
p-hydroxy benzoic acid) which used in food and personal care products as a preservative.
It is applied in the production of liquid crystal polymers.
It is also used as an intermediate of dyes, insecticides,
pharmaceutical, pesticides and
other chemical compounds.
Salicylic Acid and its derivatives are important for the preparation of other
pharmaceutical products, dyes, flavours, and preservatives.
|