|
Nonionic surfactants are surface active agents which do not dissociate into ions in aqueous solutions, unlike anionic surfactants which have a negative charge and cationic surfactants which have a positive charge in aqueous solution. Nonionic surfactants are more widely used as detergents than ionic surfactants because anionic surfactants are insoluble in many hard water and cationic surfactants are considered to be poor cleaners. In addition to detergency, nonionic surfactants show excellent solvency, low foam properties and chemical stability. It is thought that nonionic surfactants are mild on the skin even at high loadings and long-term exposure. The hydrophilic group of nonionic surfactants is a polymerized alkene oxide (water soluble polyether with 10 to 100 units length typically). They are prepared by polymerization of ethylene oxide, propylene oxide, and butylene oxide in the same molecule. Depending on the ratio and order of oxide addition, together with the number of carbon atoms which vary the chemical and physical properties, nonionic surfactant is used as a wetting agent, a detergent, or an emulsifier.
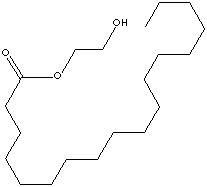
EG fatty esters are used as pearlizing agent in low viscosity shampoos, cleansing creams, liquid soaps and bath gels. Also used as an opacifier and emulsifier in lotions, and conditioners.
|
Glycol: any of a class of organic chemicals characterized by having separate two hydroxyl (-OH) groups, contribute to high water solubility, hygroscopicity and reactivity with many organic compounds, on usually linear and aliphatic carbon chain. The general formula is CnH2n(OH)2 or (CH2)n(OH)2. The wider meaning names include diols, dihydric alcohols, and dihydroxy alcohols. Polyethylene glycols and polypropylene glycols are sometimes called polyglycols which are derived by polymerization of ethylene oxide and propylene oxide respectively. Polyethylene glycols are water-soluble at all molecular weights, but polypropylene glycols become increasingly less water-soluble at high molecular weights. Ethylene glycol, HOCH2CH2OH, is the simplest member of the glycol family. Mono-, di- and triethylene glycols are the first three members of a homologous series of dihydroxy alcohols. They are colourless, essentially odourless stable liquids with low viscosities and high boiling points. Ethylene glycol is a colourless, odourless, involatile and hygroscopic liquid with a sweet taste. It is somewhat viscous liquid miscible with water boiling point 198 C, melting point 13 C soluble in ethanol, acetone, acetic acid, glycerine, pyridine, aldehydes slightly soluble in ether insoluble in oil, fat, hydrocarbones. It is prepared commercially by oxidation of ethylene at high temperature in the presence of silver oxide catalyst, followed by hydration of ethylene oxide to yield mono-, with di-, tri-, and tetraethylene glycols as co-products. The yields of ethylene glycol are depend on pH conditions. The acid-catalyzed condition in the presence of excess water provides the highest yield of monoethylene glycol. Because of its low freezing point, involatility and low corrosive activity, it is widely used in mixtures of automobile antifreeze and engine-cooling liquids. Ethylene glycol has become increasingly important in the plastics industry for the manufacture of polyester fibers and resins, including polyethylene terephthalate, which is used to make plastic bottles for soft drinks (PET bottles). MEG is the raw material in the production of polyester fiber, PET resins, alkyd, and unsaturated polyester. Diethylene glycol, CH2OHCH2OCH2CH2OH, is similar in properties to MEG, but with a higher boiling point, viscosity, and specific gravity. Diethylene glycol is used in the manufacture of unsaturated polyester resins, polyurethanes and plasticizers. It is a water-soluble liquid boiling point 245 C soluble in many organic solvents. It is used as a humectant in the tobacco industry and in the treatment of corks, glue, paper and cellophane. Diethylene glycol (DEG) is derived as a co-product with ethylene glycol and triethylene glycol. The industry generally operates to maximize MEG production. Ethylene glycol is by far the largest volume of the glycol products in a variety of applications. Availability of DEG will depend on demand for derivatives of the primary product, ethylene glycol, rather than on DEG market requirements. Triethylene glycol, HO(C2H4O)3H, is a colourless, odourless, non-volatile, and hygroscopic liquid. It is characterised by two hydroxyl groups along with two ether linkages, which contribute to its high water solubility, hygroscopicity, solvent properties and reactivity with many organic compounds. DEG is used in the synthesis of morpholine and 1,4-dioxane. TEG is displacing diethylene glycol in many of these applications on account of its lower toxicity. TEG finds use as a vinyl plasticizer, as an intermediate in the manufacture of polyester resins and polyols, and as a solvent in many miscellaneous applications. Triethylene glycol (TEG) is derived as a coproduct in the manufacture of ethylene glycol from ethylene oxide, and from " on-purpose" TEG production using diethylene glycol. Some capacities are based on total capacity for ethylene glycols. The main uses for TEG depend upon its hygroscopic properties. Air conditioning systems use TEG as dehumidifiers and, when volatilized, as an air disinfectant for bacteria and virus control. Glycols, having high boiling point and affinity for water, are employed as liquid desiccant for the dehydration of natural gas. The dehydration means the removal of water vapor in refinery tower so that dry hydrocarbon gases can exit from the top of the tower. There are wide range of glycol ethers which have bifunctional nature of ether and alcohol. cellosolves are monoether derivatives of ethylene glycol. They are excellent solvents, having solvent properties of both ethers and alcohols. Glycol family products are versatile compounds used in the fields include
- Anti-freezing and anti-icing additive
- Intermediate in polymer production and chemical reaction
- Solvent or plasticizer for plastic, lacquer, paint and varnish
- Hydraulic, brake, thermal exchange fluids and fuel additive
- Humidifying and plasticizing
- Dehydrating
- Coupling printing inks
-
Textile conditioning
- Solvent for dyes in textile and leather finishing
- Agricultural formulation
- General purpose cleaners
- Explosives manufacture
- Electrolytic component
- Humectant
- Water-based coating
- Preservative, rust remover, and disinfectant
|