| CAS
NO. |
105-56-6 |
|
| EINECS NO. |
203-309-0 |
| FORMULA |
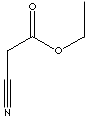
NCCH2COOC2H5 |
| MOL
WT. |
113.12 |
|
H.S.
CODE
|
2926.90 |
|
TOXICITY
|
Oral
rat LD50: 2820 mg/kg |
| SYNONYMS |
Malonic Ethyl Ester Nitrile;
|
|
Acetic
acid, cyano-, ethyl ester; Cyanessigsäureethylester
(German); Cyanoacétate d'éthyle
(French); Cianoacetato di etile (Italian);
|
| SMILES |
|
|
CLASSIFICATION
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES
|
| PHYSICAL
STATE |
clear to very light yellow
liquid |
| MELTING POINT |
-22
C |
| BOILING
POINT |
209
C |
| SPECIFIC GRAVITY |
1.063
|
| SOLUBILITY
IN WATER |
Insoluble
|
| pH |
|
| VAPOR DENSITY |
|
|
AUTOIGNITION
|
478
C
|
|
NFPA
RATINGS
|
|
|
REFRACTIVE
INDEX
|
1.4175
|
| FLASH
POINT |
110
C
|
| STABILITY |
Stable
under ordinary conditions. Color turns
dark upon exposure to light |
|
APPLICATIONS
|
Nitrile is any
of a family of organic compounds containing cyano group (-C》N) which is attached
to a carbon atom and having the general formula RC》N. Their names are
corresponding to carboxylic acids by changing '-ic acid' to '-onitrile', or
'-nitrile', whichever preserves a single letter o. Examples are acetonitrile
from acetic acid and benzonitrile from benzoic acid. Pendant nitriles are often
named as ^cyano ̄ substituents. Cyanoacetic acid, the half
nitriled-malonic acid, and its esters are basic chemical intermediates for
the production of;
- Malonates
- Barbitals
- Caffeine,
Betaine, Vitamin B
- Pharamceuticals
- Glycine
- Surfactancts
- Agrochemicals
- Dyestuffs
- Adhesives
- Indigo
dyes
- Herbicides
- Engineering plastics
|
| SALES
SPECIFICATION |
|
APPEARANCE
|
Clear
to very yellow liquid
|
|
ASSAY
|
99.0%
min
|
|
WATER
|
0.1%
max
|
|
ACID
|
30
mg/kg (as
cyanoacetic acid)
|
|
COLOR,
APHA
|
50
max
|
| TRANSPORTATION |
| PACKING |
200kgs
in drum |
| HAZARD CLASS |
6.1
(Packing group : III) |
| UN
NO. |
2810
|
| GENERAL
DESCRIPTION OF NITRILE |
|
Nitrile is an organic compounds containing cyano group (-C》N, containing
trivalent nitrogen) which is attached to one carbon atom with the general
formula RC》N. Their names are corresponding to carboxylic acids by changing '-ic
acid' to the suffix, '-onitrile' which denotes only the 》N atom (triply bound)
excluding the carbon atom attached to it, or the suffix, '-carbonitrile' where
the carbon atom in the -CN is included, whichever preserves a single letter O.
Examples are acetonitrile from acetic acid and benzonitrile from benzoic acid.
The prefix, 'cyano-' is used as an alternative naming system to indicate the
presence of a nitrile group in a molecule for the compounds of salts and
organic derivatives of hydrogen cyanide (HC》N). Isocyanides are salts and hydrocarbyl
derivatives from the isomer, HN+》C-.
Sodium cyanide, NaCN; potassium
cyanide, KCN; calcium cyanide, Ca(CN)2; and hydrocyanic (or prussic) acid, HCN
are examples.
Although nitriles don't have a carbonyl group, they are often considered as
derivatives of carboxylic acids. Nitrile undergoes acid hydrolysis to form a
carboxylic acid. Nitrile is reduced to form amine in the presence of nickel
catalyst. Grignard reagents add to nitriles, forming a relatively stable imino
derivative which can be hydrolyzed to a ketone. Metaloimine is hydrolyzed to
give beta-ketoester. Nitrile undergoes a sequence of nucleophilic additions with
an alcohol under acid catalysis, called nitrile alcoholysis. Nitriles are
hydrolyzed to carboxylic acids, alcoholyzed to esters, reduced to amines,
cyclyzed to pyridine derivatives.
|
