PRODUCT IDENTIFICATION
693-33-4
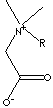
211-748-4
H.S. CODE
2923.90
TOXICITY
Oral rat LD50: 1620 mg/kg
CLASSIFICATION
SURFACTANTS /
PHYSICAL AND CHEMICAL PROPERTIES (30% AQ. SOLUTION)
MELTING POINT
SOLUBILITY IN WATER
8.5 - 9.5 (10% Aq. solution)
2,500 - 3,000 cps at 25 C
REFRACTIVE INDEX
APPLICATIONS
Amphoteric surfactants have dual functional groups (both acidic and basic groups) in the same molecule. They are polar solvents that have a high solubility in water but a poor solubility in most organic solvents. They are electrically neutral but carries positive and negative charges on different atoms in an aqueous solution. Depending on the composition and conditions of pH value, the substances can have anionic or cationic properties. In the presence of acids, they will accept the hydrogen ions but they will donate hydrogen ions to the solution in the presence of bases, which balances the pH. Such actions make buffer solutions which resist change to the pH. In the detergency ability amphoteric surfactants which change their charge according to the pH of the solution affects properties of foaming, wetting and detergentcy through a surface action that exerts both hydrophilic and hydrophobic properties. In biochemistry amphoteric surfactant is used as a detergent for purifying, cleansing and antimicrobial effects. Alkylbetains and aminoxides are amphoteric surfactants.
Members of amphoteric surfactants
- 2-Alkyl-N-carboxymethyl-N-hydroxyethyl imidazolinium betaine
- Alkyldimethyl amine oxide
- Cocoamine oxide
- Cocoamphocarboxyglycinate
- Cocoamphopolycarboxyglycinate
- Cocodimethylamine oxide
- Cocoiminoglycinate
- Cocoiminopropionate
- Cocoylamidepropyldimethyl grycine
- Lauryl betaine
- Lauryldimethyl amine oxide
- Laurylhydroxy sulfobetain
- Laurylamide propyldimethyl grycine
- Lauryldimethyl betaine
- Octyliminodipropionate
- Oleylamphopolycarboxyglycinate
- Stearyl betaine
- Tallowamine oxide
- Tallowamphopolycarboxyglycinate
- Tetradecyldimethyl amine oxide
ACTIVE MATTER
25.0% min
TOTAL SOLIDS
30.0% min
SODIUM CHLORIDE
5.5% max
pH
8.5 - 9.5 (10% Aq. solution)
COLOR (GARDNER)
2 max