ANTHRANILAMIDE
PRODUCT IDENTIFICATION
88-68-6
H.S. CODE
TOXICITY
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
590 C
185 C
APPLICATIONS
Intermediate for dyes and pharmaceuticals
APPEARANCE
ASSAY (G.C)
99.0% min
MOISTURE
1.0% max
not regulated
|
|
|
|
ANTHRANILAMIDE |
||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. |
88-68-6 |
|
| EINECS NO. | 201-851-2 | |
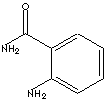
| FORMULA | 2-(H2N)C6H4CONH2 | |
| MOL WT. | 136.15 | |
|
H.S. CODE |
|
|
|
TOXICITY |
||
| SYNONYMS | o-Aminobenzamide; ATA; Anthranilic Amide; | |
| 2-Carbamoylaniline; | ||
| PRICE | ||
|
CLASSIFICATION |
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE | light brown powder | |
| MELTING POINT | 107 - 110 C (Decomposes) | |
| BOILING POINT | 300 C | |
| SPECIFIC GRAVITY |
|
|
| SOLUBILITY IN WATER | Soluble in hot water | |
| pH | ||
| VAPOR DENSITY | ||
| AUTOIGNITION |
590 C |
|
| NFPA RATINGS | Health: 1; Flammability: 1; Reactivity: 0 | |
| FLASH POINT |
185 C |
|
| STABILITY | Stable under normal conditions | |
|
APPLICATIONS |
||
|
Intermediate for dyes and pharmaceuticals |
||
| SALES SPECIFICATION | ||
|
APPEARANCE |
light brown powder | |
|
ASSAY (G.C) |
99.0% min |
|
|
MOISTURE |
1.0% max |
|
| TRANSPORTATION | ||
| PACKING | 25kgs in bag | |
| HAZARD CLASS |
not regulated |
|
| UN NO. | ||
| OTHER INFORMATION | ||
| Hazard Symbols: n/a, Risk Phrases: n/a, Safety Phrases: 24/25/28A/37/45 | ||
| GENERAL DESCRIPTION OF AMIDE | ||
| Amide is a group of organic chemicals with the general formula RCO-NH2 such as acetamide, where 'R' groups range from hydrogen to various linear and ring structures or a compound with a metal replacing hydrogen in ammonia such as sodium amide, NaNH2. Amide is formed from of ammonia (NH3) and a carboxylic acid in which a carbon atom is solid bonded to oxygen and also to an hydroxyl group or by reaction of an acid chloride, acid anhydride, or ester with an amine. An amide is hydrolyzed to yield an amine and a carboxylic acid. The reverse of this process results in the loss of water and is used in nature to link amino acids to form proteins, the secondary structure of which is due in part to the hydrogen bonding abilities of amides. Sulfonamides are analogs of amides in which the atom solid bonded to oxygen is sulfur rather than carbon. Polyamide is a polymer containing repeated amide groups, as in various kinds of nylon and polyacrylamides. | ||
|
|
|