|
AMMONIUM LAURYL SULFATE | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 2235-54-3 |
|
| EINECS NO. | 218-793-9 | |
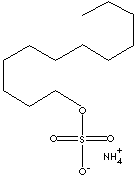
| FORMULA | C12H29NO4S | |
| MOL WT. | 283.43 | |
|
H.S. CODE |
||
|
TOXICITY |
||
| SMILES |
|
|
| CLASSIFICATION |
|
|
| SYNONYMS | Ammonium dodecyl sulfate; | |
| Sulfuric acid, monododecyl ester, ammonium salt; Dodecyl ester of sulfuric acid, ammonium salt; Dodecyl sulfate ammonium salt; Ammoniumdodecylsulfat (German); Sulfato de amonio y dodecilo (Spanish); Sulfate d'ammonium et de dodécyle (French); | ||
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE | light yellow Viscous Liquid | |
| MELTING POINT | ||
| BOILING POINT | > 100 C | |
| SPECIFIC GRAVITY |
0.998 | |
| SOLUBILITY IN WATER | ||
| pH | 5 - 10 (1% sol.) | |
| VAPOR DENSITY | ||
| AUTOIGNITION |
| |
| NFPA RATINGS |
| |
|
REFRACTIVE INDEX |
| |
| FLASH POINT |
> 93 C | |
| STABILITY | Stable under ordinary conditions | |
|
APPLICATIONS |
||
| Sulfonic acid is a compound with general formula RSO2OH, where R is an aliphatic or aromatic hydrocarbon. It is a derivative of sulfuric acid (HOSO2OH) where an OH has been replaced by a carbon group or a compound where a hydrogen atom has been replaced by treatment with sulfuric acid; for example, benzene is converted to benzenesulfonic acid (water-soluble). Sulfonic acid has a sulfur atom bonded to a carbon atom of a hydrocarbon and bonded also to three oxygen atoms, one of which has been attached to a hydrogen atom. Sulfonic acid is acidic due to the hydrogen atom, stronger than a carboxylic acid. Sulfonic acid is one of the most important organo sulfur compounds in organic synthesis. Sulfonic acids are used as catalysts in esterification, alkylation and condensation reactions. Sulfonates are salts or esters of sulfonic acid. Sulfonic salts are soluble in water. Sulfonic acid and its salts present in organic dyes provide useful function of water solubility and or improve the washfastness of dyes due to their capabiltity of binding more tightly to the fabric. They are widely used in the detergent industry. Alkylbenzene sulfonic acid is the largest-volume synthetic surfactant because of its relatively low cost, good performance, the fact that it can be dried to a stable powder and the biodegradable environmental friendliness. Sodium lauryl sulfate (SLS), prepared by sulfation of lauryl alcohol and neutralisation with sodium carbonate, is another common surfactant which has an amphiphilic properties due to C12 chain ( lipophilic) attached to a sulfate group (hydrophilic). This bifunctionality in one molecule provides the basic properties useful in cleaners and detergents. SLS is used as a wetting agent in textiles, foaming and cleaning agent in detergent, cosmetic emulsifier, and sometimes in toothpastes. Sulfonate cleaners do not form an insoluble precipitates in hard water. Ammonium lauryl sulfate (ALS) is a structurally related compound, replacing ammonium group for sodium. They have same applications. But they cause skin and eye irritation, and are therefore not useful in in products that are on the skin for a long time. The ethoxylated SLS and ALS are less irritant on the skin; sodium laureth sulfate (sodium lauryl ether sulfate, SLES) and ammonium laureth sulfate (ammonium lauryl ether sulfate, ALES) which are prepared by addition of ethylene oxide. SLES and ALES are used as a foaming and viscosity builder in shampoos and personal care products (such as bubble bath, shaving cream , ointment, and tooth pastes sometimes) particularly of low pH products. One more common feature of them appears to be the compatibility with other surfactants. | ||
| SALES SPECIFICATION | ||
|
APPEARANCE |
light yellow viscous liquid | |
| ACTIVE MATTER | 28.0 ± 1% | |
|
INORGANIC SALT |
1.0% max | |
| UNSULPHONATED WT |
1.0% max | |
|
pH |
5 - 10 (1% sol.) | |
| WATER |
5.0 - 7.0% | |
| TRANSPORTATION | ||
| PACKING | 200kgs in plastic Drum | |
| HAZARD CLASS | ||
| UN NO. | ||
| OTHER INFORMATION | ||
|
|
||