PRODUCT IDENTIFICATION
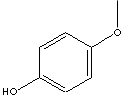
H.S. CODE
TOXICITY
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
MELTING POINT
SOLUBILITY IN WATER
REFRACTIVE INDEX
NFPA RATINGS
Health: -, Flammability: 1, Reactivity: 0
AUTOIGNITION
133
APPLICATIONS
APPEARANCE
99.0% min
WATER
0.2% max
Quinone is a group of aromatic compounds containing two opposite carbonyl groups (C=O) and the other two pairs of carbon atoms linked by vinylene group(-CH=CH) in a six-membered unsaturated ring. The carbonyl groups are located in different rings and form various chemical structures which offer important roles to colours. Quinones are used in photography and dye manufacture. Quinones occur benzoquinones, naphthoquinones, anthraquinones, and polycyclic quinones. Though quinones are found in plants and in a few animals, they usually are prepared by oxidation of aromatic amines, polyhydric phenols, and polynuclear hydrocarbons.The reduction of quinone to the corresponding dihydroxy form is an important characteristic reaction. In acidic solution, p-benzoquinone is reduced reversibly to hydroquinone. The so-called quinhydrone electrode, containing equimolar solution of quinone and hydroquinone, is used to determine hydrogen ion concentrations depends on the oxidation-reduction reactions. Hydroquinone and its derivatives used principally in photographic dye chemicals, in medicine, as an antioxidant, and in paints, varnishes, and motor fuels and oils. Hydroquinone and certain derivatives are also used as polymerization inhibitors by direct reacting with peroxy-free radical to tie up free radicals.