|
Phosphonates derived from phosphorous (phosphonic) acid are employed in the
applications of scale Inhibition, sequestration, dispersion and corrosion
inhibition in addition to the main applications of agricultural chemicals such
as fertilizers, pesticides, and soil conditioners. Phosphonates offer a wide
range of sequestrants to control metal ions in aqueous systems. By forming
stable water soluble complexes with multivalent metal ions, phosphonates prevent
undesired interaction by blocking normal reactivity of metal ions. This ability
contributes to function as threshold industrial water treatment and metal
treatment processes (antiscalants, corrosion inhibitors, chelants, sludge
conditioners, pulp bleachings, deflocculants, dispersants, metal cleaners,
electroplating and crystal growth modifiers). Phosphonates are also used in
manufacturing detergents, cosmetics and personal care products for special
functions such as low levels iron control, stain removal, bleach stabilization,
peroxide stabilization and anti-encrustation. Phosphonates existing in various
compounds as acids or salts are marketed in the form of concentrated solutions.
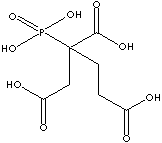
PBTC
has three carboxy groups in one molecule. This multifunctional carboxylic acid
provides covalently linked structure with hydroxy group. Carboxylic acids form amide derivatives.
PBTC is a raw material to produce aromatic polyimide
resins having high resistance to thermal stresses. It is a metal chelator. It is
used as a corrosion inhibitor and a metal-cleaning composition. It is used as a chemical intermediate.
Its derivatives, acyl halides, anhydrides, esters, amides and nitriles, are
used in making target products such as flavoring agents,
pesticides, cosmetic ingredients, dyes, textile treatment agents, fungicides, and pharmaceuticals
through further reactions of substitution, catalytic reduction, metal hydride
reduction, diborane reduction, keto formation with organometallic reagents,
electrophile bonding at oxygen, and Claisen condensation on carboxylic group.
|
|
Carboxylic acid is an organic compound whose molecules contain carboxyl group
and have the condensed chemical formula R-C(=O)-OH in which a carbon atom is
bonded to an oxygen atom by a solid bond and to a hydroxyl group by a single
bond), where R is a hydrogen atom, an alkyl group, or an aryl group. Carboxylic
acids can be synthesized if aldehyde is oxidized. Aldehyde can be obtained by
oxidation of primary alcohol. Accordingly, carboxylic acid can be obtained by
complete oxidation of primary alcohol. A variety of Carboxylic acids are
abundant in nature and many carboxylic acids have their own trivial names.
Examples are shown in table. In substitutive nomenclature, their names are
formed by adding -oic acid' as the suffix to the name of the parent compound.
The first character of carboxylic acid is acidity due to dissociation into H+
cations and RCOO- anions in aqueous solution. The two oxygen atoms are
electronegatively charged and the hydrogen of a carboxyl group can be easily
removed. The presence of electronegative groups next to the carboxylic group
increases the acidity. For example, trichloroacetic acid is a stronger acid than
acetic acid. Carboxylic acid is useful as a parent material to prepare many
chemical derivatives due to the weak acidity of the hydroxyl hydrogen or due to
the difference in electronegativity between carbon and oxygen. The easy
dissociation of the hydroxyl oxygen-hydrogen provide reactions to form an ester
with an alcohol and to form a water-soluble salt with an alkali. Almost infinite
esters are formed through condensation reaction called esterification between
carboxylic acid and alcohol, which produces water. The second
reaction theory is the addition of electrons to the electron-deficient carbon
atom of the carboxyl group. One more theory is decarboxylation (removal of
carbon dioxide form carboxyl group). Carboxylic acids are used to synthesize
acyl halides and acid anhydrides which are generally not target compounds. They
are used as intermediates for the synthesis esters and amides, important
derivatives from carboxylic acid in biochemistry as well as in industrial
fields. There are almost
infinite esters obtained from carboxylic
acids. Esters
are formed by removal of water from an acid and an alcohol. Carboxylic acid
esters are used as in a variety of direct and indirect applications. Lower chain
esters are used as flavouring base materials, plasticizers, solvent carriers and
coupling agents. Higher chain compounds are used as components in metalworking
fluids, surfactants, lubricants, detergents, oiling agents, emulsifiers, wetting
agents textile treatments and emollients, They are also used as intermediates
for the manufacture of a variety of target compounds. The almost infinite esters
provide a wide range of viscosity, specific gravity, vapor pressure, boiling
point, and other physical and chemical properties for the proper application
selections. Amides
are formed from the reaction of a carboxylic acids with an amine.
Carboxylic
acid's reaction to link amino acids is wide in nature to form proteins (amide), the
principal constituents of the protoplasm of all cells. Polyamide is a polymer
containing repeated amide groups such as various kinds of nylon and
polyacrylamides. Carboxylic acid
are in our lives. |