PRODUCT IDENTIFICATION
H.S. CODE
TOXICITY
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
MELTING POINT
SOLUBILITY IN WATER
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
APPLICATIONS
APPEARANCE
80% min
108 C (10 hr)
OXYGEN CONTENT
10% min
|
|
|
| 2,5-DIMETHYL-2,5-BIS(T-BUTYLPEROXY)HEXANE | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 78-63-7 |
|
| EINECS NO. | 201-128-1 | |
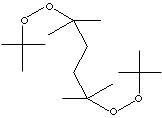
| FORMULA | [(CH3)3COOC(CH3)2CH2-]2 | |
| MOL WT. | 290.45 | |
|
H.S. CODE |
2909.60 | |
|
TOXICITY |
||
| SYNONYMS | 2,5-Bis(tert-butylperoxy)-2,5-dimethylhexane; Luperox 101; | |
| 2,5-Dimethyl-2,5-di(tertiary-butylperoxy)-hexane; Kayahexa AD; 2,5-Dimethyl-2,5-Bis(tert-Butylperoxy)hexane; Trigonox 101; Varox 50; (1,1,4,4-tetramethyl-1,4-butanediyl)bis((1,1-dimethylethyl) Peroxide; | ||
| PRICE | ||
|
CLASSIFICATION |
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE | clear to yellow liquid | |
|
MELTING POINT |
8 C | |
| BOILING POINT | ||
| SPECIFIC GRAVITY | 0.865 | |
|
SOLUBILITY IN WATER |
Insoluble | |
| pH | ||
| VAPOR DENSITY | ||
|
AUTOIGNITION |
| |
|
NFPA RATINGS |
||
|
REFRACTIVE INDEX |
1.4185 | |
| FLASH POINT |
| |
| STABILITY | Stable under ordinary conditions. oxidizing chemical | |
|
APPLICATIONS |
||
| 2,5-Dimethyl-2,5-bis(t-butylperoxy)Hexane is used to control melt index in polypropylene polymerization processing and monomer scavenger styrene polymerization processing. It is used as free radical polymerization initiator, vulcanizing agent, crosslinking agent and as a chemical intermediate. | ||
| SALES SPECIFICATION | ||
|
APPEARANCE |
yellow oily liquid | |
| PURITY |
80% min | |
| HALF LIFE |
108 C (10 hr) | |
|
OXYGEN CONTENT |
10% min | |
| TRANSPORTATION | ||
| PACKING | 25kgs in drum | |
| HAZARD CLASS | ||
| UN NO. | 2156 | |
| GENERAL DESCRIPTION OF PEROXIDE | ||
| Peroxide: Compound containing the peroxy group (-O-O-), chainlike structure, containing two oxygen atoms, each of which is bonded to the other and to a radical or some element. It is considered that hydrogen peroxide is the starting material to prepare organic and inorganic peroxides commercially. Hydrogen Peroxide H2O2, is a powerful oxidizing agent. The most valuable property of hydrogen peroxide is that it breaks down into water and oxygen and therefore does not form any persistent, toxic residual compounds. It is used in the processes of epoxidation, oxidation, hydroxylation and reduction. Its oxidizing properties are used in the bleachings and deodorizing for textile, hair and in paper manufacture. It is also used medicinally as an antiseptic. Its application involves the production of chemicals like perhydrates as well as organic peroxides in which some organic (or inorganic) substituents have replaced one or both hydrogens. Some metals form peroxides in air sodium, barium or zinc. Metal peroxide releases oxygen slowly in contact with atmospheric moisture and used to as disinfectants in cosmetics, detergents, toothpaste and pharmaceuticals. They can be used in the bleachings and deodorizing and a oxygen release source in agricultural application to generate contaminated soils and lakes. Organic Peroxides are powerful oxidizing agents releasing oxygen. They are widely used as initiators,catalysts and crosslinking agent for the polymerization process in the plastics manufacturing industry and as chemical intermediates, bleaching agents, drying and cleaning agents. They are also used as antiseptics, disinfectants and germicides medically for cosmetics, detergents, toothpaste and pharmaceuticals. Organic peroxides include peroxyacetic acid, benzoyl peroxide, cumyl and tert-butyl peroxides. | ||
|
|
|