PRODUCT IDENTIFICATION
H.S. CODE
UN NO.
TOXICITY
CLASSIFICATION
EXTRA NOTES
PHYSICAL AND CHEMICAL PROPERTIES
MELTING POINT
SOLUBILITY IN WATER
REFRACTIVE INDEX
NFPA RATINGS
Health: 1, Flammability: 1, Reactivity: 0
AUTOIGNITION
421 C
216 C
EXTERNAL LINKS & GENERAL DESCRIPTION
Wikipedia Linking (Hydroquinone)
Local:
Antioxidant is a substance added in small quantities to hydrocarbons which are
susceptible to oxidation, such as rubbers, plastics, foods, and oils to inhibit
or slow oxidative processes, while being itself oxidized. Antioxidants work in
two different ways. In primary antioxidants (also called free-radical
scavengers), antioxidative activity is implemented by the donation of an
electron or hydrogen atom to a radical derivative. These antioxidants are
usually hindered amines (p-Phenylene diamine, trimethyl dihydroquinolines,
alkylated diphenyl amines) or substituted phenolic compounds with one or more
bulky functional groups such as a tertiary butyl at 2,6 position commonly.
Butylated hydroxytoluene (BHT) is a common example of hindered phenolic
antioxidant. The reaction rate, or carbocation stability, in SN1 mechanism is 3° > 2° > 1° > CH3
(no SN1) so, tertiary alkyl moiety exists in lots of phenolic antioxidant
compounds. Primary antioxidants are free radical scavengers which combine with
peroxy radicals and break autocatalytic cycle. In secondary antioxidants ( also
called peroxide decomposers), activity is implemented by the removal of an
oxidative catalyst and the consequent prevention of the initiation of oxidation.
Examples of peroxide decomposer type of antioxidant are trivalent phosphorous
and divalent sulfurcontaining compound such as sulfides, thiodipropionates and
organophosphites. Synergistic effect is expected when primary antioxidants are
used together with secondary antioxidants as primary antioxidants are not very
effective against the degradation by UV oxidation. Sometimes, chelating agents
are added to scavenge metal impurities which can initiate decomposition.
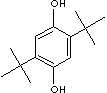
DTBHQ is used as an inhibitor, antioxidant, and stabilizer. It is useful as an antioxidant for rubber products, a stabilizer, and an anti-skinning agent in paints.
APPEARANCE
98.0% min
WATER
0.1% max