|
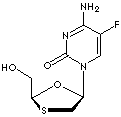
EMTRICITABINE |
| Synonyms. Emtricitabine; (-)-2'-Deoxy-5-fluoro-3'-thiacytidine; (-)-FTC; (2R-cis)-4-Amino-5-fluoro-1-(2-(hydroxymethyl)-1,3-oxathiolan-5-yl)-2(1H)-pyrimidinone; 2'-Deoxy-5-fluoro-3'-oxa-4'-thiocytidine; 2'-Deoxy-5-fluoro-3'-thiacytidine; 2-FTC; 5-Fluoro-1-((2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl)cytosine; Coviracil; Emtriva; FTC; dOTFC; |
|
|
| PRODUCT IDENTIFICATION |
|
|
CAS RN |
143491-57-0 |
|
EINECS RN |
|
|
FORMULA |
C8H10FN3O3S |
|
MOLE WEIGHT |
247.25 |
|
H.S CODE |
|
|
SMILES |
S1C[C@H](O[C@H]1CO)n1cc(c(nc1=O)N)F |
|
CLASSIFICATION |
Anti-Infective, Antiretroviral, Antiviral, Deoxycytidine nucleoside analog, Pyrimidone, Oxathiolane, Organofluoride |
|
EXTRA NOTES |
Antiviral [treatment of HIV-1 and hepatitis B infections] |
|
|
| PHYSICAL AND CHEMICAL PROPERTIES |
|
|
PHYSICAL STATE. |
white to off-white crystalline powder |
|
MELTING POINT |
219 - 220 C |
|
BOILING POINT |
440 C |
|
DENSITY |
1.82 |
|
SOLUBILITY IN WATER |
|
| SOLVENT SOLUBILITY | |
|
VAPOR DENSITY |
|
|
log P(octanol-water) |
|
|
VAPOR PRESSURE |
|
|
AUTOIGNITION TEMP |
|
| pH |
|
|
REFRACTIVE INDEX |
|
|
FLASH POINT |
221 C |
|
|
| STABILITY AND REACTIVITY | |
| STABILITY | Stable under normal conditions. |
|
INCOMPATIBLE MATERIALS |
Oxidizing agents |
| POLYMERIZATION |
Has not been reported |
|
NFPA RATINGS |
|
|
|
| EXTERNAL LINKS & GENERAL DESCRIPTION |
|
Drug Information Portal (U.S. National Library of Medicine) - Emtricitabine PubChem Compound Summary - Emtricitabine Drug Bank - Emtricitabine KEGG (Kyoto Encyclopedia of Genes and Genomes) - Emtricitabine http://www.ebi.ac.uk/chebi/ - Emtricitabine http://www.ncbi.nlm.nih.gov/ - Emtricitabine http://hivinsite.ucsf.edu/ https://www.factsandcomparisons.com/
|
|
|
| SALES SPECIFICATION |
|
|
APPEARANCE |
white crystalline powder |
|
ASSAY |
99.0% min |
|
SPECIFIC ROTATION |
-138° ~ -143° |
| RESIDUE IGNITION |
0.01% max |
| LOSS ON DRYING |
0.1% max |
| MELTING POINT |
219 - 220 C |
|
|
| TRANSPORT & REGULATORY INFORMATION |
|
|
UN NO. |
Not regulated |
| HAZARD SYMBOL |
|
| PACKING GROUP | |
|
|
| SAFETY INFORMATION |
|
|
HAZARD OVERVIEW |
Not known |
|
GHS |
|
|
SIGNAL WORD |
|
|
PICTOGRAMS |
|
|
HAZARD STATEMENTS |
|
|
P STATEMENTS |
|
| EC DIRECTIVES |
|
| HAZARD CODES |
|
|
RISK PHRASES |
|
|
SAFETY PHRASES |
|
|
|
| PACKING |
|
|
|
|
| PRICE INFORMATION |
|
|