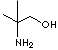
PRODUCT IDENTIFICATION
UN NO.
CLASSIFICATION
Buffer, Neutralizing agent, Amino Alcohol
EXTRA NOTES
PHYSICAL AND CHEMICAL PROPERTIES
0.93
REFRACTIVE INDEX
67 C
EXTERNAL LINKS & GENERAL DESCRIPTION
http://www.sigmaaldrich.com/
Application: Used in an efficient synthesis of 2-oxazolidinones via
carbonylation with CO in the presence of salen-cobalt catalysts. Used to
derivatize carboxylic acids for GC analysis and to synthesize 2-oxazolines for
further transformations.
http://www.sigmaaldrich.com/
Useful
pH Ranges of Selected Biological Buffers
http://www.scifun.org/
Chemical
of the Week -- Biological Buffers
Many chemical reactions
are affected by the acidity of the solution in which they occur. In order for a
particular reaction to occur or to occur at an appropriate rate, the pH of the
reaction medium must be controlled. Such control is provided by buffer
solutions, which are solutions that maintain a particular pH. Biochemical
reactions are especially sensitive to pH. Most biological molecules contain
groups of atoms that may be charged or neutral depending on pH, and whether
these groups are charged or neutral has a significant effect on the biological
activity of the molecule. In all multicellular
organisms, the fluid within the cell and the fluids surrounding the cells have a
characteristic and nearly constant pH. This pH is maintained in a number of
ways, and one of the most important is through buffer systems. Two important
biological buffer systems are the dihydrogen phosphate system and the carbonic
acid system.
APPEARANCE
99.0% min
0.5% max