|
NYSTATIN |
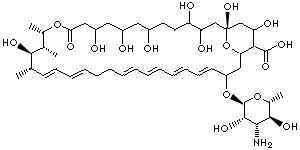
| Comycin; Myco-Triacet II; Mycolog II; Mytrex; Nystaform; Nystaform-HC; Terrastatin; Biofanal; Candex; Diastatin; Fungicidin; Herniocid; Korostatin; Moronal; Myconystatin; Mycostatin; Mykinac; Mykostatyna; Nilstat; Nistatin; Nistatina; Nistatina; Nyamyc; Nyotran; Nystan; Nystatin; Nystatine; Nystatinum; Nystatyna; Nystatyna; Nystavescent; Nystex; Nystop; O-V Statin; Stamycin; Zydin E; (1S,15S,16R,17R,18S,19E,21E,25E,27E,29E,31E)-33-[(2S,3S,4S,5S,6R)-4-amino-3,5-dihydroxy- 6-methyloxan-2-yl]oxy-1,3,4,7,9,11,17,37-octahydroxy-15,16,18-trimethyl-13-oxo-14, 39-dioxabicyclo[33.3.1]nonatriaconta-19,21,25,27,29, 31-hexaene-36-carboxylic acid |
|
|
| PRODUCT IDENTIFICATION |
|
|
CAS RN |
1400-61-9 |
|
EINECS RN |
215-749-0 |
|
FORMULA |
C47H75NO17 |
|
MOLE WEIGHT |
926.09 |
|
H.S.CODE |
2941.90.1010 |
|
CLASSIFICATION |
Polyene antifungal, Antibacterial, Anti-Infective, Ionophore |
|
EXTRA NOTES |
Macrolide antifungal antibiotic complex produced by Streptomyces noursei, S. aureus, and other Streptomyces species. The biologically active components of the complex are nystatin A1, A2, and A3. Used as a fungal membrane (ergosterol binding) pore forming agent and to create nystatin/ergosterol based ion channels in lipid bilayers. Used as a lipid raft-inhibiting reagent and membrane associated cholesterol. Mode of Action: Increases the permeability of the cell membrane of sensitive fungi by binding to sterols. Antimicrobial spectrum: Yeasts and molds. |
|
|
| PHYSICAL AND CHEMICAL PROPERTIES |
|
|
PHYSICAL STATE |
white to off-white powder |
|
MELTING POINT |
|
|
BOILING POINT |
|
|
DENSITY |
|
|
SOLUBILITY IN WATER |
Partially soluble (360 mg/l) |
|
pH |
|
|
VAPOR DENSITY |
|
|
REFRACTIVE INDEX |
|
|
FLASH POINT |
|
|
|
| STABILITY AND REACTIVITY | |
| STABILITY | Stable under normal conditions. |
|
INCOMPATIBLE MATERIALS |
Strong oxidizing agents |
| DECOMPOSITION PRODUCTS |
Carbon oxides, nitrogen oxides |
| POLYMERIZATION | Has not been reported |
|
NFPA RATINGS |
Health:1 , Flammability:0 , Reactivity:0 |
|
|
| SAFETY |
|
|
HAZARD NOTES |
|
|
EYE |
May cause eye irritation. |
|
SKIN |
May be harmful if absorbed through skin. May cause skin irritation. |
|
INGESTION |
May be harmful if swallowed. |
|
INHALATION |
May be harmful if inhaled. May cause respiratory tract irritation. |
|
CHRONIC |
|
|
|
| TRANSPORT & REGULATORY INFORMATION |
|
|
UN NO. |
|
| HAZARD CLASS |
|
| PACKING GROUP |
|
| HAZARD SYMBOL |
XI |
|
RISK PHRASES |
36/37/38 |
|
SAFETY PHRASES |
26-36 |
|
|
| EXTERNAL LINKS & GENERAL INFORMATION | ||||||||||||||||||||||||||||||||||||||||||||||||
|
Nystatin is an antibiotic which is both fungistatic and fungicidal in vitro against a wide variety of yeasts and yeast-like fungi, including Candida albicans, C. parapsilosis, C. tropicalis, C. guilliermondi, C. pseudotropicalis, C. krusei, Torulopsis glabrata, Tricophyton rubrum, T. mentagrophytes. Nystatin acts by binding to sterols in the cell membrane of susceptible species resulting in a change in membrane permeability and the subsequent leakage of intracellular components. On repeated subculturing with increasing levels of nystatin, Candida albicans does not develop resistance to nystatin. Generally, resistance to nystatin does not develop during therapy. However, other species of Candida (C. tropicalis, C. guilliermondi, C. krusei, and C. stellatoides) become quite resistant on treatment with nystatin and simultaneously become cross resistant to amphotericin as well. This resistance is lost when the antibiotic is removed. Nystatin exhibits no appreciable activity against bacteria, protozoa, or viruses. Nystatin interacts with 14-α demethylase, a cytochrome P-450 enzyme necessary for the conversion of lanosterol to ergosterol. This results in inhibition of ergosterol synthesis and increased fungal cellular permeability. ( http://www.drugbank.ca/) Similar to other polyene antifungal agents, nystatin binds to ergosterol in the fungal membrane. This binding disrupts osmotic integrity of the fungal membrane, resulting in leakage of intracellular potassium, magnesium, sugars, and metabolites and then cellular death. The lipid carrier of liposomal nystatin does not change this basic mode of action ( http://www.doctorfungus.org/) Ionophore is a relatively small hydrophobic molecule that facilitates the transport of ions across lipid membranes. Most ionophores are produced by microorganisms. There are two types of ionophores: channel formers, which combine to form a channel in the membrane through which ions can flow; and mobile ion carriers, which transport ions across a membrane by forming a complex with the ion. (http://www.ktf-split.hr/)
|
|
|
| SALES SPECIFICATION |
|
|
APPEARANCE |
white to off-white powder |
|
ASSAY |
4400µg/mg min |
|
LOSS ON DRYING |
5.0% max |
| pH |
6.0 - 8.0 |
|
|
| PACKING |
|
|
|
|
| PRICE INFORMATION |
|
|