|
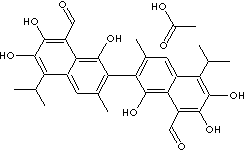
GOSSYPOL ACETIC ACID |
| 2,2'-Bis(1,6,7-trihydroxy-3-methyl-5-isopropyl-8-aldehydonaphthalene) acetic acid; 8-Formyl-1,6,7-trihydroxy-5- isopropyl- 3-methyl-2,2'-bisnaphthalene acetic acid; 1,1',6,6',7,7'- Hexahydroxy-5,5'-diisopropyl-3,3'-dimethyl -(2,2'-binaphthalene)-8,8'-dicarboxaldehyde acetic acid; 2,2'-bis(8-Formyl-1,6,7-trihydroxy-5-isopropyl-3- methylnaphthalene) acetic acid; Acetic acid gossypol; Gossypol acetate; 7-(8-Formyl-1,6,7-trihydroxy-3-methyl-5-propan-2-ylnaphthalen- 2-yl)-2,3, 8-trihydroxy- 6-methyl-4-propan-2-ylnaphthalene-1-carbaldehyde acetic acid; |
|
|
| PRODUCT IDENTIFICATION |
|
|
CAS RN |
12542-36-8 |
|
EINECS RN |
|
|
FORMULA |
C30H30O8·C2H4O2 |
|
MOLE WEIGHT |
578.61 |
|
|
| PHYSICAL AND CHEMICAL PROPERTIES |
|
|
PHYSICAL STATE |
white to yellow crystalline powder |
|
MELTING POINT |
|
|
BOILING POINT |
|
|
DENSITY |
|
|
SOLUBILITY IN WATER |
|
|
pH |
|
|
VAPOR DENSITY |
|
|
REFRACTIVE INDEX |
|
|
FLASH POINT |
|
|
|
| STABILITY AND REACTIVITY | |
| STABILITY | Stable under normal conditions |
|
INCOMPATIBLE MATERIALS |
Strong oxidizing agents. |
| DECOMPOSITION PRODUCTS |
Carbon oxides. |
| POLYMERIZATION | Has not been reported |
|
NFPA RATINGS |
Health: 1, Flammability: 0, Reactivity: 0 |
|
|
| SAFETY |
|
|
HAZARD NOTES |
Target Organ Effect. Target Organs: Male reproductive system. |
|
EYE |
May cause eye irritation. |
|
SKIN |
May be harmful if absorbed through skin. May cause skin irritation. |
|
INGESTION |
May be harmful if swallowed. |
|
INHALATION |
May be harmful if inhaled. May cause respiratory tract irritation. |
|
CHRONIC |
|
|
|
| TRANSPORT & REGULATORY INFORMATION |
|
|
UN NO. |
|
| HAZARD CLASS |
|
| PACKING GROUP |
|
| HAZARD CODE |
XN |
|
RISK STATEMENTS |
22-40 |
|
SAFETY STATEMENTS |
22-36 |
|
|
| EXTERNAL LINKS & GENERAL INFORMATION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Gossypol is a poisonous pigment found in cottonseed and its name is derived from the botanical name of the cotton plant, Gossypium L, Malvaceae. The toxicity of gossypol is shown against the reproductive system, heart, liver, and membranes. The compound exhibits both pro- and anti-oxidant behavior. Electron transfer (ET) functionalities, present in gossypol and its metabolites, comprise conjugated dicarbonyl, a quinone derivative, Schiff bases, and metal complexes. The parent possesses a reduction potential favorable for in vivo ET. Considerable evidence points to oxidative stress, formation of a reactive oxygen species, and DNA scission, characteristics of redox cycling by ET in biosystems, as mechanism of action for gossypol. In a study on the apoptotic effect of gossypol on human lymphocytes, gossypol was used at 20-50 mM to induce apoptosis in human lymphocytes without causing necrosis through cytotoxic effects. The combined use of steroid hormones (methyltestosterone and ethinyl estradiol) and gossypol (low dose) in an antifertility study in rats showed that the steroid hormone made the procedure of spermatogenesis slower and low dose gossypol caused all sperm to lose their activity in the epididymis. Both affect the process of spermatogenesis from different endpoints and successfully induce infertility in the short term. A low dose of gossypol not only executes antifertility function in the epididymis, but also affects the quality of spermatozoal production in testis by impacting the procedures of both acrosomal formation and spermatozoal elongation. This assists in maintaining long term infertility. Gossypol, as a PAF antagonist/inhibitor, markedly inhibited the contractile responses of guinea-pig lung parenchyma strips stimulated with leukotriene B4, leukotriene D4, and PAF-acether. It was suggested that the inhibition of the myotropic activity of the lung parenchyma by gossypol is due to interactions with the formation of cyclooxygenase products within the guinea-pig lung. (http://www.sigmaaldrich.com/) Gossypol is a chemical found in the seeds of cotton plants. Cotton plants produce gossypol in order to slow down the reproduction of the insects that eat cotton bolls and seeds; the compound also affects reproduction in mammals. Pressed cakes of cotton seeds, a byproduct of the cotton industry, are sometimes fed to livestock with unintentional contraceptive effects. The effect of gossypol on human male fertility has been known in China for many years. In 1929, a study of couples who used crude cottonseed oil for cooking showed that they had smaller than average families. Specifically, researchers showed that the oil affected male fertility. Eventually researchers isolated the contraceptive compound gossypol from the cotton seed oil. This discovery led to large scale testing of gossypol as a male contraceptive in China during the 1970s. The studies involved over 8,000 men, and continued for over a decade. The researchers found that men taking a daily gossypol pill had reliable contraception and no complaints about change in libido. However, the studies revealed two serious flaws: disruption of potassium uptake and incomplete reversibility. (http://www.malecontraceptives.org/) Gossypol is a dimeric or bis-naphthalene natural product isolated from the seeds of cotton plants (various Gossypium species). The naphthalene rings, however, are derived from sesquiterpenes of the cadinane family. The cadinanes are formed in the biogenetic cascade from the bisabolane intermediate by a series of putative 1,2-shifts and cyclization. The interest in gossypol derives from its activity as a contraceptive in human males. It has been shown to affect the maturation and motility of sperm, and it inactivates the enzymes required for the sperm to fertilize the ova. Gossypol has been used clinically in China and the contraceptive effect has been shown to be reversible in short term usage. Gossypol also has been found to have antiviral, antiherpes, antipsoriasis, antikeratitus, and antineoplastic activity. Gossypol exists as two atropisomers due to restricted rotation about the biaryl bond. The contraceptive effect appears to be associated with the (-)-isomer, while toxic effects (cardiac toxicity in cattle) appear to be associated with the (+)-isomer. (http://www.people.vcu.edu/) Pharmacological actions:
Contraceptive agents
|
|
|
| SALES SPECIFICATION |
|
|
APPEARANCE |
white to yellow crystalline powder |
|
ASSAY |
95.0% min (HPLC) |
|
HEAVY METAL |
20ppm max |
|
ARSENIC |
1ppm max |
|
LEAD |
2ppm max |
|
MICROBIOLOGY |
Total Plate Count:1000cfu/g Yeast and Mold: 100cfu/g E.Coli: Negative Salmonella: Negative |
|
RESIDUAL SOLVENTS |
pass EP.2000 (Water and Alcohol) |
|
SIEVE ANALYSIS |
80 mesh (100%) |
|
|
| PRICE INFORMATION |
|
|