PRODUCT IDENTIFICATION
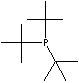
H.S. CODE
SMILES
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
clear low melting solid
102 - 103 at 13 mmHg
NFPA RATINGS
AUTOIGNITION
REFREACTIVE INDEX
APPLICATIONS
APPEARANCE
clear liquid (in 10% Hexane)
99.0% min
Phosphine, also called Hydrogen Phosphide, PH3, is a colourless, poisonous, spontaneously flammable gas, with a disagreeable, garlic-like odour melting point -133.5 C, boiling point -87.4 C. Phosphine is manufactured by the hydrolysis of metal phosphides, by the electrolysis of phosphorus in the presence of hydrogen, or by the phosphorus-steam reaction. Phosphine has the structure of ammonia (NH3) but with phosphorus in place of nitrogen. It is slightly soluble in cold water and soluble in alcohol. Phosphine is less soluble in water than ammonia. Phosphine is used in the synthesis of organophosphines and phosphonium derivatives and as a dopant in the manufacture of semiconductors. Aluminium or magnesium phosphide are used in the formulation for fumigation in pest control, and zinc phosphide is used as a rodenticide. Phophene is a starting material for the preparation of pesticides and flame retardants. Organophosphines are used as solvents for the extraction and separation processes, flame retardants, and in formulating fumigants and electronics applications of semiconductor manufacturing. Tertiary alkylphosphines act as chemical intermediate and catalyst in the production of industrial acids, alcohols, flavors & fragrances, and pharmaceuticals. Phosphonium describes a univalent radical, PH4. Quaternary phosphonium salts, obtained from tertiary alkylphosphines with the treatment with alkyl or aromatic halides, are replacing phase transfer catalysts and biocides functions for quaternary ammonium salts due to more effective performance and higher thermal stability. Phosphonium salts are used as epoxy curing agents. A variety of phosphine transition metal complexes including chiral complexes are synthesized as the very reactive and versatile not only homogeneous and heterogeneous but also stereospecific catalysts.homogeneous catalysts and stereospecific as well.