PRODUCT IDENTIFICATION
103-79-7
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
1.015
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
83 C
APPLICATIONS
Phenyl acetone is used as an intermediate to produce pesticides and anticoagulants. Active ingredients as anticoagulant include:
- Brodifacoum
- Chlorophacinone
- Coumachlor
- Difenacoum
- Diphacinane
- 2-Pivaloyl-1,3-indandione
- 2-Isovaleryl-1,3-indandione
- Warfarin
Phenyl acetone is used as an intermediate to produce sympathomimetic amines such as phenbenzamine, phenyl isopropyl amine, amphetamine, prenylamine, pargyline and estramustine. Phenylacetone is called MDP2P (3,4-methylenedioxy phenyl-2-propanone) which is used in the clandestine synthesis of MDMA (3,4-methylenedioxymethamphetamine), commonly known as ecstasy. Phenylacetone shipment is available only to the importer who has import licence issued by Government.
APPEARANCE
99.0% min
ACIDITY
Carbonyl groups are:
- Aldehydes (X and Y = H; X = H, Y = alkyl or aryl)
- Ketones (X and Y = alkyl or aryl)
- Carboxylic acids (X = OH, Y = H, alkyl, or aryl)
- Esters (X = O-alkyl or aryl; Y = H, alkyl, or aryl)
- Amides (X = NH, N-alkyl, or N-aryl; Y = H, alkyl, or aryl)
- Acid halides
- Acid anhydrides
- Lactones
- Lactams
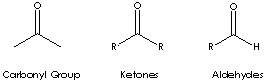
Ketone has the general formula RCOR' where the groups R and R' may be the same or different, or incorporated into a ring (R and R' are alkyl, aryl, or heterocyclic radicals). The simplest example, R and R´ are methyl group, is acetone (also called 2-propanone, CH3COCH3) which is one of the most important ketones used in industry (low molecular weight ketones are general purpose solvents.) In the IUPAC system, the suffix -one is used to describe ketone with the numbering of the carbon atom at the end that gives the lower number. For example, CH3CH2COCH2CH2CH3 is named 3-hexanone because the whole chain contains six carbon atoms and the oxygen is connected to the third carbon from the lower number. There are aromatic ketones of which acetophenone and bezophenone are examples. Ketones can be made by the oxidation of secondary alcohols and the destructive distillation of certain salts of organic acids. In addition to as polar solvents, ketones are important intermediates in the syntheses of organic compounds such as alkoxides, hydroxyalkynes, imines, alcohols (primary, secondary as well as tertiary), acetals, thioacetals, phosphine oxides, geminal diols, hydrazones, organic sulfite and cyanohydrins.