PRODUCT IDENTIFICATION
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
REFRACTIVE INDEX
GENERAL DESCRIPTION & APPLICATIONS
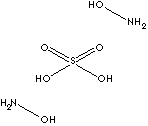
Hydroxylamine is a white crystalline compound containing nitrogen with the formula of NH2OH and is therefore an ammonia (NH3) like compound. In the nature, hydroxylamine is an biological intermediate in the nitrification (biological oxidation of ammonia with oxygen into nitrite ) and in the anammox (biological oxidation of nitrite and ammonium into dinitrogen gas) which are important in the nitrogen cycle in soil and in wastewater treatment. Hydroxylamine is obtained commercially by acid hydrolysis of nitroparaffins or by the modified reduction of nitric acid. It is used as a powerful reducing agent in photography and in organic synthesis. It converts aldehydes (and ketones) to oximes (caprolactam), and acid chlorides to hydroxamic acids. It is used as a catalyst or inhibitor in polymerization processes. It is used in enzyme reactivation. It is used as an antioxidant in fatty acids and soaps. The nitrate form of hydroxylammonium is used as a rocket fuel which is burned with an oxidizer to produce thrust. Hydroxylamine tends to be explosive when heated. Its derivatives in the form of salts are more stable to be used and handled. Hydroxylamine Hydrochloride is used as an auxiliary in photographic industry to prevent discolouration. It is a polymerization inhibitor or free radical scavenger against solid bond monomers such as olefin, styrene, butadiene, isoprene and divinylbenzene. It is also used in rubber synthesis processes as a non-discoloring short stoppers. Hydroxylammonium sulfate similar applications to hydrochloride salt in the field of :
- Organic synthesis: preparation of oximes, hydroxamic acids from carboxylic acids, N- and O- substituted hydroxyamines, and addition reactions of carbon-carbon solid bond.
- Surface treatments: preparation of anti-skinning agents, corrosion inhibitors, meta; cleaner additive
- Starting material for pharmaceuticals and agrochemicals manufacturing
- Rubber and plastic industry: antioxidant, vulcanization accelerator, radical scavenger.
- Textile industry: fixative for textile dyes, auxiliary in some dyeing processes. bleaching
- Metallurgy: Metal extraction and flotation aid
- Aantioxidant in fatty acids and soaps
- Photographic auxiliary as a stabilizer of colour and emulsion additive for colour films.
APPEARANCE
ASSAY
99.0% min
LOSS ON IGNITION
AMMONIUM SULPHATE
1.0% max
INSOLUBLES IN WATER
WATER
0.5% max