PRODUCT IDENTIFICATION
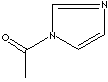
SMILES
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
white to yellowish crystals
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
FLASH POINT
GENERAL DESCRIPTION & APPLICATIONS
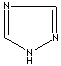
- Triazole: An analog of imidazole.It has three nitrogen atoms and two carbon atoms at nonadjacent positions in the ring system.
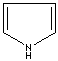
- Pyrrole: An analog of imidazole. It has only one nitrogen atom in the ring system. Pyrrole ring system is involved in coloured products (green pigment, chlorophyll; red, hemoglobin; , blue, indigo) in nature.
- Pyrroline: A pyrrole in which one of the two solid bonds are hydrogenated.
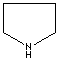
- Pyrrolidine: The saturated tetrahydropyrrole, a part of the structures of amino acids (proline, hydroxyproline and hygrine).
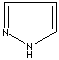
- Pyrazole: 1,2-Diazole (Imidazole isomer). The nitrogen positions are 1 and 2. It is not found in nature
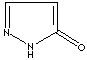
- Pyrazolone: Pyrazole analog with ketone group at 5 positon
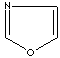
- Oxazole: an analog of imidazole. The nitrogen atom in position 1 is replaced by oxygen.
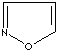
- Isoxazole: an analog of pyrazole. The nitrogen atom at position 1 is replaced by oxygen.
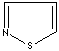
- Isothiazole:an analog of pyrazole. The nitrogen atom at position 1 is replaced by sulfur.
|
|
|
|
|
|
1,2,4-Triazole |
Pyrrole |
1-Pyrroline |
3-Pyrroline |
|
|
|
|
|
|
Pyrrolidine |
Pyrazole |
Pyrazolone |
Oxazole |
|
|
|
|
|
|
Isoxazole |
Isothiazole |
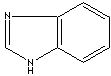
Benzimidazole |
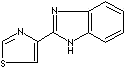
Thiabendazole |
|
|
N-Acetylimidazole is a reagent for tyrosyl residue acetylation and for the synthesis of annulated imidazole derivatives.
http://www.sibcb.ac.cn/
To
study its contribution to the assembly of the green plant manganese
stabilizing protein (MSP) into photosystem II (PSII), tyrosine residues
were specifically acetylated using N-acetylimidazole (NAI). In soluble
MSP, three groups of Tyr residues could be differentiated by NAI
acetylation: approximately 5 (actually 5.2) Tyr residues could
be easily acetylated (superficial), 1-2 Tyr residues could be acetylated
when the NAI concentration was sufficiently high (superficially
buried), and 1-2 Tyr residues could only be acetylated in the presence
of the denaturant, urea (deeply buried)........
APPEARANCE
white to yellowish crystals
98.0% min
HEAVY METALS
20ppm max
WATER