PRODUCT IDENTIFICATION
547-58-0 (Sodium)
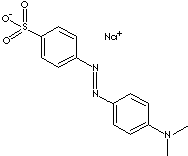
c1(ccc(cc1)S(=O)(=O)[O-])/N=N/c1ccc(cc1)N(C)C.[Na+]
CLASSIFICATION
Acid Dye, Stain,
EXTRA NOTES
PHYSICAL AND CHEMICAL PROPERTIES
REFRACTIVE INDEX
EXTERNAL LINKS & GENERAL DESCRIPTION
Wikipedia Linking - Methyl Orange
Google Scholar Search - Methyl Orange
Drug Information Portal (U.S. National Library of Medicine) - Methyl Orange
PubChem Compound Summary - Methyl Orange
KEGG (Kyoto Encyclopedia of Genes and Genomes) - Methyl Orange
http://www.ebi.ac.uk/ - Methyl Orange
http://www.ncbi.nlm.nih.gov/ - Methyl Orange
http://toxnet.nlm.nih.gov/
Hazardous
Substances Data Bank - Methyl Orange
Local:
Acid dyes are water-soluble dyes employed mostly in the form of sodium salts of
the sulfonic or carboxylic acids. They are anionic which attach strongly to
cationic groups in the fibre directly. They can be applicable to all kind of
natural fibres like wool, cotton and silk as well as to synthetics like
polyesters, acrylic and rayon. But they are not substantive to cellulosic
fibres. They are also used in paints, inks, plastics and leather. Orange acid
azo dyes produce an orange-pink color. They are used in coloring foods and drugs
and as intermediates for making photosensive dyes and drugs. They are used as
counter stains in histology and cytology and as components of Mallory's acid
fuchsin. Methyl Orange (acid magenta) is a various mixture of sulfonated fuchsins.
There are four of these compounds which have three sulfonic groups each. It is
used in Andrade's indicator and in various complex stains to demonstrate
collagen fibres red and in smooth muscle in contrast to
collagen. It is used as a pH indicator. The
pH range of Methyl Orange id form pH 3.0 pink-red to pH 4.4 yellow.
Methyl Orange
is used in oil field industry for alkalinity test of mud filtrate.
Examples of orange acid dyes
- Ethyl orange Sodium salt ( CAS RN: 62758-12-7)
- Orange II ( CAS RN: 633-96-5)
- Orange III (methyl orange, CAS RN: 547-58-0)
- Orange IV (CAS RN: 554-73-4)
- Orange G ( CAS RN: 1936-15-8)
- Tropaeolin O (CAS RN: 547-57-9)
- Victoria orange
- Acid Orange II (Dibromofluorescein, 596-03-2)
APPEARANCE
95.0% min
LOSS ON DRYING
5.0% max
(pH 4.4)467-471nm: 750 - 850
HAZARD OVERVIEW
Toxic by ingestion
GHS
PICTOGRAMS

HAZARD STATEMENTS
H301
P STATEMENTS
P301 + H310
![]()
RISK PHRASES
25
SAFETY PHRASES
46
BIOLOGICAL STAINS, INDICATORS