PRODUCT IDENTIFICATION
CLASSIFICATION
EXTRA NOTES
PHYSICAL AND CHEMICAL PROPERTIES
REFRACTIVE INDEX
EXTERNAL LINKS & GENERAL DESCRIPTION
Wikipedia Linking - Benzenesulfonic acid
Drug Information Portal (U.S. National Library of Medicine) - Metanilic acid
PubChem Compound Summary - Metanilic acid
KEGG (Kyoto Encyclopedia of Genes and Genomes) - Metanilic acid
http://www.ebi.ac.uk/ - Metanilic acid
http://www.ncbi.nlm.nih.gov/ - Metanilic acid
http://www.clri.org/
Biodegradation
of studies on Metanilic acid:
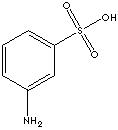
Synonyms : 3-Aminobenzenesulfonic
acid, Aniline, Meta Sulphonic Acid.
Metanilic acid is an isomer
of sulfanilic acid with the sulfonic acid group at position 2. Hence,
162��M concentration of Metanilic acid degradation studies was carried
out in O.curviceps BDU 92191 to further confirm the biodecolourization
potential of the marine cyanobacterium.
http://www.1911encyclopedia.org/
Benzene
sulphonic acid, C6H 5 S0 3 H,11H 2 O, crystallizes in small plates
and is very deliquescent. Benzene sulphochloride, C6H5 S02C1, is
a colourless fuming liquid which boils at 120° C. (10 mm.).
The aminobenzene sulphonic acids, particularly the meta and para
compounds, are of importance owing to their employment in the colour
industry. The direct sulphonation of aniline yields the para acid,
sulphanilic acid, C 6 H 4 (NH 2)(SO 3 H), which crystallizes in
small plates and is sparingly soluble in cold water. When fused
with caustic potash it yields aniline, whilst oxidation with chromic
acid yields benzoquinone. In constitution it is probably to be regarded
?NH3 as a cyclic ammonium salt, C 6 H 4 When diazotized in NS0 3
/ . acid solution and coupled with dimethyl aniline it yields helianthine,
the sodium salt of which is used as an indicator. Metanilic acid
C6H4(NH2) (S03H) [1.3], which crystallizes in prisms, is formed
b y the reduction of meta-nitrobenzene sulphonic acid and is used
in the preparation of various azo dyes.
Local:
Sulfanilic acid is a light grey powder or crystals; slightly soluble in water,
alcohol, and ether, soluble in hot water and fuming hydrochloric acid, char at
288 - 300 C. Sulfanilic acid is a sulfonated aniline product. Aniline is the
starting material in the dye manufacturing industry. Sulfonic acid and its salts present in organic dyes provide useful function of
water solubility and or improve the washfastness of dyes due to their
capabiltity of binding more tightly to the fabric. Sulfanilic acid is used as an intermediate for colorants (dyes, food colors,
optical brightening agents), medicines and other organic synthesis. It is a
component of griess reagent to determine nitrous acid. Sulfanilic acid is converted
to sulfanilamide which is one of the basic materials
to produce antibacterial sulfa drugs. There is an isomer
called metanilic acid, sulfonic group at position 2.
It is used in the
manufacture of azo dyes and in synthesis sulfa drugs.
APPEARANCE
99.0% min
HAZARD OVERVIEW
May cause methemoglobinemia. Harmful if swallowed, inhaled, or absorbed through the skin. Causes burns by all exposure routes. Target Organs: Blood, blood forming organs, eyes, skin, mucous membranes
GHS
PICTOGRAMS

HAZARD STATEMENTS
H302-H312-H332
P STATEMENTS
P280
![]()
RISK PHRASES
20/21/22
SAFETY PHRASES
23-28
PRICE INFORMATION