PRODUCT IDENTIFICATION
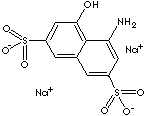
5460-09-3 (Sodium Salt)
2922.21
1-Amino-8-hydroxynaphthalene-3,6-disulfonic acid Monosodium Salt; 1-Amino-8- Naphthol-3,6-Disulfonic Acid; 4-Amino-5-hydroxy- 2,7-naphthalene Disulfonic acid, monosodium salt; 8-Amino-1-naphthol-3,6-disulfonic acid monosodium salt monohydrate;
Naphthalene
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
APPLICATIONS
Naphthols are either of two crystalline monohydric alcohols, derived from naphthalene and belonging to the phenol family. The naphthols are position isomers; Alpha-naphthol (also called 1-naphthol) is 1-hydroxynaphthalene and Beta-naphthol (also called beta-naphthol) is 2-hydroxynaphthalene. The compound 1-naphthol is made by heating 1-naphthalenesulfonic acid with caustic alkali or by heating 1-naphthylamine with water under pressure. The compound 2-naphthol is manufactured by fusing 2-naphthalenesulfonic acid with caustic soda. They are used directly in making several dyes and are converted into numerous dye intermediates, as well as into tanning agents, antioxidants, and antiseptics.
Sulfonic acid is a compound with general formula RSO2OH, where R is an aliphatic or aromatic hydrocarbon. It is a derivative of sulfuric acid (HOSO2OH) where an OH has been replaced by a carbon group or a compound where a hydrogen atom has been replaced by treatment with sulfuric acid; for example, benzene is converted to benzenesulfonic acid (water-soluble). Sulfonic acid has a sulfur atom bonded to a carbon atom of a hydrocarbon and bonded also to three oxygen atoms, one of which has been attached to a hydrogen atom. Sulfonic acid is acidic due to the hydrogen atom, stronger than a carboxylic acid. Sulfonic acid is one of the most important organo sulfur compounds in organic synthesis. Sulfonic acids are used as catalysts in esterification, alkylation and condensation reactions. Sulfonates are salts or esters of sulfonic acid. Sulfonic salts are soluble in water. Sulfonic acid and its salts present in organic dyes provide useful function of water solubility and or improve the washfastness of dyes due to their capabiltity of binding more tightly to the fabric. They are widely used in the detergent industry. Alkylbenzene sulfonic acid is the largest-volume synthetic surfactant because of its relatively low cost, good performance, the fact that it can be dried to a stable powder and the biodegradable environmental friendliness. Sulfonate cleaners do not form an insoluble precipitates in hard water. Sulfonic acid salts and esters are intermediates widely used in organic synthesis and particularly phenolic compounds and cation exchange resins. They are synthetic intermediate for a number of biologically active compounds and pharmaceutical candidates such as sulfa drugs. Short carbon chain metallic sulfonate is used in electroplating which is a process for applying a metallic coating on a metal surface by electrodeposition from a suitable electrolyte solution for imparting corrosion resistance and direct production of printed circuit boards without etching out of a piece of copper sheet.
H-Acid (1-amino-8-hydroxynaphthalene-3,6-disulfonic acid), an important dye intermediate, is produced from naphthalene by a combination of the unit processes of sulfonation, nitration, reduction, and hydrolysis. H Acid is used in the manufacture of a large number of azo dyes and pigments.
APPEARANCE
85.0% min
0.2% max (in soda sol.)
MOISTURE
5.0% max
CHROMOTROPIC ACID
2.0% max
KOCH'S ACID
0.5% max
PRICE