PRODUCT IDENTIFICATION
3.0 - 4.6
CLASSIFICATION
Dyes, Indicator
EXTRA NOTES
PHYSICAL AND CHEMICAL PROPERTIES
EXTERNAL LINKS & GENERAL DESCRIPTION
Local: The relationship between the activity of hydrogen ions [exactly hydronium ions, H(H2O)n+] and concentration of a solution is fundamentally important to determine the extent of a chemical reaction, as it affects the equilibria and kinetics of a wide variety of chemical and biochemical reactions. The hydrogen-ion activity refers to the effective concentration of unassociated hydrogen ions, the form that directly affects physicochemical reaction rates and equilibria. The symbol, pH, numerically relates the hydrogen ions concentration or activity. The pH is approximately equal to the negative logarithm of H+ concentration expressed in molarity. pH 7 is neutral; above it alkalinity increases and below it acidity increases. pH indicators are usually weak acidic or basic organic tautomers which exist in more than one structural form of which at least one form is characteristically colored in relation to different electronic configuration of the bound. Indicators should not change color exactly at one pH value, but within a wide pH range. The transition point of an indicator is defined as the point at which the acid and alkaline forms of the indicator exist in equal concentrations. Visual transition intervals are as below:
- alpha-Naphthol phthalein (CAS #: 596-01-0): from pH 7.3 (pinkish-yellow) to pH 8.7 (greenish-blue)
- Bromocresol green (CAS #: 76-60-8): from pH 3.8 (yellow) to pH 5.4 (blue)
- Bromcresol Purple (CAS #: 115-40-2): from pH 5.2 (yellow) to pH 6.8 (purple)
- Bromochlorophenol Blue (CAS #: 2553-71-1): from pH 3.2 (yellow) to pH 4.8 (blue)
- Bromocresol purple, sodium salt (CAS #: 62625-30-3): from pH 5.2 (yellow) to pH 6.8 (purple)
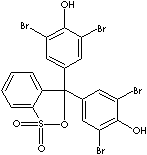
- Bromophenol blue (CAS #: 115-39-9): from pH 3.0 (yellow) to pH 4.6 (blue)
- Bromophenol blue, sodium salt (CAS #: 62625-28-9): from pH 3.0 (yellow) to pH 4.6 (blue)
- Bromopyrogallol Red (CAS #: 16574-43-9): suitable for complexometric or chelometric titrations
- Bromothymol blue (CAS #: 76-59-5): from pH 6.0 (yellow) to pH 7.6 (blue)
- Bromothymol blue, sodium salt (CAS #: 34722-90-2): from pH 6.0 (yellow) to pH 7.6 (blue)
- Bromoxylenol Blue (CAS #: 40070-59-5): from pH 6.0 (yellow) to pH 7.6 (blue)
- Chlorophenol Red, water soluble (CAS #: 123333-64-2): from pH 4.6 (yellow) to pH 7.0 (purple)
- Cresol Red (CAS #: 1733-12-6): from pH 7.0 (orange) to pH 8.8 (purple)
- Fluorescein (CAS #: 2321-07-5)
- Fluorescein sodium (CAS #: 518-47-8):
- Glycinethymol Blue (CAS #: 3810-63-7):
- Iodophthalein sodium (CAS #: 2217-44-9)
- m-Cresol purple (CAS #: 2303-01-7): from pH 7.4 (yellow) to pH 9.0 (purple)
- Methylthymol blue, sodium salt (CAS #: 1945-77-3): suitable for complexometric or chelometric titrations
- o-Cresolphthalein (CAS #: 596-27-0): from pH 8.2 (colorless) to pH 9.8 (red)
- o-Cresolphthalein Complexone (CAS #: 2411-89-4): suitable for complexometric or chelometric titrations
- Phenolphthalein Complexone (CAS #: 25296-54-2):
- Phenol Red (CAS #: 143-74-8): from pH 6.8 (yellow) to pH 8.2 (red)
- Phenol Red Sodium Salt (CAS #: 34487-61-1): from pH 6.8 (yellow) to pH 8.2 (red)
- Phenolphthalein (CAS #: 77-09-8): from pH 8.5 (colorless) to 9.0 (red)
- p-Xylenol Blue (CAS #: 125-31-5):
- Acid range: from pH 1.2 (red) to pH 2.8 (yellow)
- Alkali range: from pH 8.0 (yellow) to pH 9.6 (purplish-blue)
- Pyrocatechol violet (CAS #: 115-41-3): suitable for determination of many metals (complexometric or chelometric titrations) particularly rapid for tin
- Pyrogallol Red (CAS #: 32638-88-3):
- Thymol Blue, sodium salt (CAS #: 62625-21-2):
- Acid range: from pH 1.2 (red) to pH 2.8 (yellow)
- Alkaline range: from pH 8.0 (yellow) to pH 9.2 (blue)
- Thymolphthalein Complexone (CAS #: 1913-93-5): suitable for complexometric or chelometric titrations
- Thymolphthalein (CAS #: 125-20-2): from pH 8.8 (colourless) to pH 10.5 (blue)
Phthalein is the moiety in a dye which shows color changes depend on particular hydrogen ion concentrations. Certain phthaleins (e.g. phenolphthalein) have a cathartic action.
Bromocresol Green, 3',3'',5',5''-Tetrabromo-m-cresolsulfonephthalein, is a pH used as an indicator form pH 3.8 (yellow) and 5.4 (blue). It is a brown powder sparingly soluble in water, soluble in benzene, and very soluble in ethanol and diethyl ether. The sodium salt is a dark green powder. Both of free acid and sodium salt are used as pH indicator. It was used in serum albumin determinations. Bromocresol Green is used in oil field industry for alkalinity test of mud filtrate. Bromocresol Purple is 5',5''-Dibromo-o-cresolsulfonephthalein, a yellow powder soluble in water. The pH range is from pH 5.2 (yellow) to pH 6.8 (purple). Bromophenol red (CAS RN: 2800-80-8) is 5',5''-Dibromophenolsulfonphthalein, a brown powder with pH range from 5.2 (orange-yellow) to pH 6.8 (red). Bromophenol Blue, 3',3'',5',5''-Tetrabromophenolsulfonephthalein, is used as a pH indicator form 3.0 (yellow) to 4.6 (blue). Both free acid and sodium salts are used as a tracking dye for nucleic acid or protein electrophoresis in agarose or polyacrylamide gels. It is used in detal cleaning.
APPEARANCE
DYE CONTENT
~ 95.0%
SOLUTION CLARITY
Pass