PRODUCT IDENTIFICATION
21282-97-3
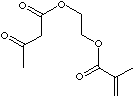
214.21
H.S. CODE
TOXICITY
Oral rat LD50:>5000 mg/kg
2-(Acetoacetoxy)ethyl methacrylate; AAEM;
2-(Methacryloyloxy)-ethyl acetoacetate; Acetoacetic acid, 2-hydroxyethyl-ester methacrylate; Butanoic acid, 3-oxo-, 2-((2-methyl-1-oxo-2-propenyl)oxy) ethylester; Other CAS RN: 135623-38-0; 50981-32-3;
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
clear to yellowish liquid
MELTING POINT
SOLUBILITY IN WATER
REFRACTIVE INDEX
1.4560
Health: 1, Flammability: 1, Reactivity: 0
106 C
APPLICATIONS
Acetoacetates have a reactive hydrogen atom on the carbon alpha to both carbonyl groups. It undergoes Knoevenagel condensation reaction as a reactant to forms a large class of target products including amino acids, drugs, colorants, lacquers, perfumes, and plastics. Alone, it is used as a flavoring agent and a solvent. Knoevenagel condensation is a nucleophilic addition of a reactive hydrogen atom at 1,3-diketone compounds to a carbonyl group, followed by an dehydration reaction. 1,3-Diketone compounds (beta-ketones) include malonic acid, diethyl malonate, Meldrum's acid, and acetoacetic acid derivatives.
Acetoacetoxyethyl Methacrylate is used as a comonomer in polimerization as a viscosity reducer for adhesives, varnishes, polyesters and coatings. It is used as an additive in polymer.
APPEARANCE
clear to yellowish liquid
ASSAY
95.0% min
ACID VALUE
3.0 - 8.0 (mg KOH/g)