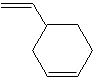
PRODUCT IDENTIFICATION
CLASSIFICATION
EXTRA NOTES
PHYSICAL AND CHEMICAL PROPERTIES
0.8263
REFRACTIVE INDEX
16 C
EXTERNAL LINKS & GENERAL DESCRIPTION
http://monographs.iarc.fr/
Production:
4-Vinylcyclohexene is isolated as a by-product of other production
processes involving 1,3-butadiene (see IARC, 1992), including the
production of vinylnorbornene, dodecanedioic acid and 1,3-butadiene
itself. The 4-vinylcyclohexene isolated in these processes may be
sold or converted to the diepoxide, or may be recycled for further
use within the production facility (Che mi cal Manufacturers Association,
1991). 4-VinylcycIohexene is prepared commercially by catalytic
dimerization of 1,3-butadiene at LLO-425°C and 1.3-100 MPa.
The catalysts used are tyically silicon carbide and copper or chromium
salts (Kirshenbaum, 1978). No data were available to the Working
Group on production volume. Use: 4-Vinylcyclohexene has been used
as an intermediate for producing flame retardants, flavours and
fragrances; in the manufacture of polyolefins; as a solvent; and
in the manufacture of special chemicals such as the diepoxide (see
monograph, p. 361) (DuPont Chemicals, undated).
Local:
Vinyl: the univalent chemical radical H2C=CHCl-, derived from ethylene. It is a
highly reactive, easily polymerizing, and low cost monomer used as basic
materials for one of largest-selling plastic. In addition to its application of
polymerization to make plastics with huge amount, vinly- is an functional group
involved in cycloaddition, addition reactions, and carbon skeleton
expansion reactions including Suzuki reaction, Heck reaction.
This radical is useful in biomolecules chemistry such
as protein sequencing and enzyme inhibitors.
4-Vinyl-1-Cyclohexene is a polymerizable monomer and a co-monomer in polymerization of other monomers useful as a chemical intermediate applied for the wide range of products; e.g cyclohexene dioxide, flame retardants through halogenation, flavors, fragrances , insecticidal compounds with ethylene chlorohydrin, diepoxides, solvents, and in other organic synthesis.
APPEARANCE
97.0% min
0.2% max