PRODUCT IDENTIFICATION
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
humid dark powder
GENERAL DESCRIPTION AND APPLICATIONS
APPEARANCE
humid dark powder
80.0%
|
|
|
| 4-NITROSOPHENOL | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 104-91-6 |
|
| EINECS NO. | 203-251-6 | |
| FORMULA | C6H5NO2 | |
| MOL WT. | 123.11 | |
| H.S. CODE | ||
| TOXICITY | ||
| SYNONYMS | Quinone Monoxime; Quinone Oxime; Nitrosophenol; | |
| p-Benzoquinone monoxime; | ||
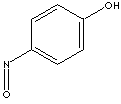
| SMILES |
|
|
|
CLASSIFICATION |
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE |
humid dark powder |
|
| MELTING POINT | > 100 C (Decomposes) | |
| BOILING POINT | ||
| SPECIFIC GRAVITY |
|
|
| SOLUBILITY IN WATER | ||
| AUTOIGNITION |
|
|
| pH | ||
| VAPOR DENSITY | ||
| NFPA RATINGS | ||
| REFRACTIVE INDEX | ||
| FLASH POINT |
|
|
| STABILITY | Stable under ordinary conditions | |
|
GENERAL DESCRIPTION AND APPLICATIONS |
||
| Nitroso is the prefix for NO- radical with trivalent nitrogen also known as hydroximino; oximido. Substances in which this group is attached to an oxygen atom are called nitrites (esters of nitrous acid); those in which the nitroso group is attached to a metal ion are called nitrosyls. Nitroso (-N=O) groups and azo (-N=N-) groups impart colour to the compound as these groups absorb light of characteristic wavelengths. Nitroso groups are strongly electron withdrawing and more similar to the C=O than the nirto group. Nitroso compound tends to dimerise. There is polarisation of the N=O bond and so behaves as a weak C=O. It undergoes addition of nucleophiles and condensation with primary amines and the anions of active methylene compounds. Nitroso groups are useful in addition and condensation reactions, stable nitroxide radical preparations, and isomerization to oxime. Oxidation undergoes readily with peracids, this is not available with nitro groups. Nitroso compounds are useful intermediates for the synthesis of target materials. Certain niroso derivatives are used as vulcanization accelerator by direct reacting with peroxy-free radical to tie up free radicals and adhesion promoter between metal and rubber. Mononitrophenols are used as intermediates for the synthesis of a number of organophosphorus pesticides, azo dyes, and some medical products as well as their corresponding amonophenols by reduction. some end products derived from mononitrophenols include dyes and pigments, acetaminophen (from p-aminophenol), carbofuran, phosalon (insecticides form o-nitrophenol), parathion,parathion-methyl, fluorodifen (insecticides form p-nitrophenol), nitrofen, bifenox (herbicides form p-nitrophenol), fungicides, and rubber chemicals. Nitrophenol is used as a pH range indicator (colorless at 5 and yellow at 7). Nitrophenol derivatives are used as intermediates for the synthesis of a number of target products. 4-Nitrosophenol is an intermediate for the preparation of sulfur containing vat dyes and sulfide dyes. | ||
| SALES SPECIFICATION | ||
|
APPEARANCE |
humid dark powder |
|
| CONTENT |
80.0% |
|
| TRANSPORTATION | ||
| PACKING | 50kgs in drum | |
| HAZARD CLASS | ||
| UN NO. | ||
| OTHER INFORMATION | ||
| Hazard Symbols: XN, Risk Phrases: 21/22-40-41, Safety Phrases: 15-24/25-26 | ||
|
|
|