PRODUCT IDENTIFICATION
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
0.81 - 0.82
AUTOIGNITION
440 C
NFPA RATINGS
REFRACTIVE INDEX
49 C
APPLICATIONS
APPEARANCE
99.0% min
|
|
|
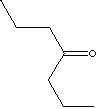
| 4-HEPTANONE | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 123-19-3 |
|
| EINECS NO. | 204-608-9 | |
| FORMULA | (C3H7)2CO | |
| MOL WT. | 114.19 | |
| H.S. CODE | 2914.19 | |
| TOXICITY |
| |
| SYNONYMS | Butyrone; Dipropyl ketone; Di-n-propyl ketone; | |
| Propyl Ketone; Heptan-4-one; | ||
| RAW MATERIALS |
|
|
|
CLASSIFICATION |
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE | colorless to pale yellow liquid | |
| MELTING POINT | -33 C | |
| BOILING POINT | 144 - 146 C | |
| SPECIFIC GRAVITY |
0.81 - 0.82 | |
| SOLUBILITY IN WATER | 4.6 (g/l) | |
| pH | 6 - 7 | |
| VAPOR DENSITY | ||
|
AUTOIGNITION |
440 C | |
|
NFPA RATINGS |
Health: 1 Flammability: 2 Reactivity: 0 | |
|
REFRACTIVE INDEX |
1.407 | |
| FLASH POINT |
49 C | |
| STABILITY | Stable under ordinary conditions. | |
|
APPLICATIONS |
||
| 4-Heptanone is used as a solvent and an intermediate in the synthesis of pharmaceuticals and pesticides. | ||
| SALES SPECIFICATION | ||
|
APPEARANCE |
colorless to pale yellow liquid | |
| PURITY |
99.0% min | |
| MOISTURE | 0.5% max | |
| TRANSPORTATION | ||
| PACKING | 160kgs in drum | |
| HAZARD CLASS | 3 (Packing group: II) | |
| UN NO. | 2710 | |
| OTHER INFORMATION | ||
| Hazard Symbols: XN , Risk Phrases: 10-11, Safety Phrases: 24/25 | ||
| GENERAL DESCRIPTION OF KETONE | ||
|
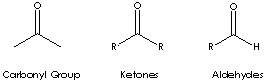
Ketone is a class of chemical compounds contain the carbonyl group in which the
carbon atom is covalently bonded to an oxygen atom. It has the general formula
RCOR' where the groups R and R' may be the same or different, or incorporated
into a ring (R and R' are alkyl, aryl, or heterocyclic radicals). The simplest
example, R and R´ are methyl group, is acetone (also called 2-propanone,
CH3COCH3) which is one of the most important ketones used in industry (low
molecular weight ketones are general purpose solvents.) In the IUPAC system, the
suffix -one is used to describe ketone with the numbering of the carbon atom at
the end that gives the lower number. For example, CH3CH2COCH2CH2CH3 is named
3-hexanone because the whole chain contains six carbon atoms and the oxygen is
connected to the third carbon from the lower number. Ketones can be made by the
oxidation of secondary alcohols and the destructive distillation of certain
salts of organic acids. Carbonyl group structures are:
|
||
|
|
|