PRODUCT IDENTIFICATION
H.S.CODE
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
APPLICATIONS
APPEARANCE
98.5% min
PRICES
|
|
|
| 4-ANISIDINE-3-SULFONIC ACID | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 13244-33-2 |
|
| EINECS NO. | 236-224-2 | |
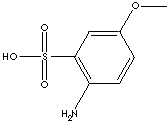
| FORMULA | H3COC6H3(NH2)SO2OH | |
| MOL WT. | 203.21 | |
|
H.S.CODE |
||
| TOXICITY | Oral rat LD50: 10 gm/kg. | |
| SYNONYMS | 2-Amino-5-methoxybenzenesulphonic acid; | |
| 2-Amino-5-methoxybenzolsulfonsäure (Dutch); ácido 2-amino-5- metoxibencenosulfónico (Spanish); Acide 2-amino-5-méthoxybenzènesulfonique (French); 4-Methoxy-2-Sulfoaniline; | ||
| SMILES | ||
|
CLASSIFICATION |
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE | light beige powder | |
| MELTING POINT | ||
| BOILING POINT | ||
| SPECIFIC GRAVITY |
| |
| SOLUBILITY IN WATER | slightly soluble | |
| pH | ||
| VAPOR DENSITY | ||
| AUTOIGNITION |
| |
| NFPA RATINGS |
Health: 2; Flammability: 0; Reactivity: 0 | |
REFRACTIVE INDEX |
||
| FLASH POINT |
| |
| STABILITY | Stable under ordinary conditions. | |
|
APPLICATIONS |
||
| Ether is any of a number of organic compounds characterized by an oxygen atom joined with single bonds by two carbon atoms that are part of hydrocarbon groups. The general formula is R-O-R', where R and R' are alkyl or aromatic groups. Ethers are formed by the condensation of two alcohols by heating with sulfuric acid; the reaction is one of dehydration. Ethers can be prepared from alkyl halide reacted with metallic alkoxide (called Williamson synthesis). Ethers are similar to alcohols but are generally less dense, less soluble in water, and have lower boiling points. They are relatively unreactive, which makes them valuable solvents. But ethers will be cleaved at high temperatures by concentrated hydrogen halides. Ethers have relatively low boiling point compare to alkanes as they don't form hydrogen bonds each other. Ethers are more lipophilic than esters [R-C(=O)-O-R']or amides [RCO-NH2]. Ethers are widely used as solvents for various organic reactions because they are relatively the least reactive among common organic compounds except alkanes and fluorocarbons. The common reaction of ethers is cleavage of the C–O bond by strong acids either in linear chain or cyclic structure. Ethers in which oxygen is bonded to primary and secondary alkyl groups can form peroxide compounds in the presence of gaseous oxygen due to two unpaired electrons in oxygen. Ethers can act as Lewis bases in chemical reactions. Commonly, ethers are named simply in listing the alkyl groups in alphabetical order or alkane order such as ethyl methyl ether or methyl ethyl ether, which is methoxyethane in IUPAC nomenclature ( the formula of "alkoxyalkane" ). When ether is a parts of complex molecule or aromatic derivatives, it is described as an alkoxy substituent such as methoxybenzene ( trivial name is anisole). The methoxy prefix indicates the function methyl group joined by single bonds to an oxygen atom, with the general formula -O-CH3. Cyclic ethers have ring structure where the oxygen has become part of the ring. The term of epoxide indicate three membered cyclic ether (also called oxirane) in which an oxygen atom is joined to each of two carbon atoms that are already bonded to each other; four membered cyclic ether is called oxetane; five membered cyclic ether, furan (or oxolane); six membered cyclic ether, pyran (also called oxane) respectively. Their unhindered oxygen atom carries two unshared pairs of electrons - a structure which favors the formation of coordination complexes and the solvation of cations. Cyclic ethers are used as important solvents, as chemical intermediate and as monomer for ring-opening polymerization. Crown Ether is a macrocyclic polyether whose structure contains hydrogen, carbon and oxygen atoms. Each oxygen atoms are confined between two carbon atoms and exhibits a conformation with a hole (accordingly called "crown"). Anisole is one of the simplest aromatic compound to which ether group is linked. But it is different with aromatic compounds like furan where the oxygen is a part of the ring. Anisole, C6H5OCH3 (methyl phenyl ether), is a clear liquid that is soluble in ether and alcohol; insoluble in water; boiling point 155 C. Anisole and its derivatives are used as solvents and in perfumery. Anisole can be obtained from anise seed. Anisic acid, p-methoxybenzoic acid, is a part of cresol class antiseptic compounds. It is also used as an insect repellent and ovicide. Anisole, anisic acid, and their derivatives are also widely used in chemical reaction as intermediates to obtain target materials such as dyes, pharmaceuticals, perfumes, photoinitiators and agrochemicals. Anisidines, methoxyanilines, are used as intermediates for the synthesis of azo dyes, pigments and other chemical compounds. Sulfonic acid is a compound with general formula RSO2OH, where R is an aliphatic or aromatic hydrocarbon. It is a derivative of sulfuric acid (HOSO2OH) where an OH has been replaced by a carbon group or a compound where a hydrogen atom has been replaced by treatment with sulfuric acid; for example, benzene is converted to benzenesulfonic acid (water-soluble). Sulfonic acid has a sulfur atom bonded to a carbon atom of a hydrocarbon and bonded also to three oxygen atoms, one of which has been attached to a hydrogen atom. Sulfonic acid is acidic due to the hydrogen atom, stronger than a carboxylic acid. Sulfonic acid is one of the most important organo sulfur compounds in organic synthesis. Sulfonic acids are used as catalysts in esterification, alkylation and condensation reactions. Sulfonates are salts or esters of sulfonic acid. Sulfonic salts are soluble in water. Sulfonic acid and its salts present in organic dyes provide useful function of water solubility and or improve the washfastness of dyes due to their capabiltity of binding more tightly to the fabric. They are widely used in the detergent industry. Alkylbenzene sulfonic acid is the largest-volume synthetic surfactant because of its relatively low cost, good performance, the fact that it can be dried to a stable powder and the biodegradable environmental friendliness. Sulfonate cleaners do not form an insoluble precipitates in hard water. Sulfonic acid salts and esters are intermediates widely used in organic synthesis and particularly phenolic compounds and cation exchange resins. They are synthetic intermediate for a number of biologically active compounds and pharmaceutical candidates such as sulfa drugs. | ||
| SALES SPECIFICATION | ||
|
APPEARANCE |
orange to red powder | |
| ASSAY (BY MASS) |
98.5% min | |
| INDIVIDUAL IMPURITY | 0.1% max | |
| TRANSPORTATION | ||
| PACKING | 25kgs in bag | |
| HAZARD CLASS | ||
| UN NO. | ||
| OTHER INFORMATION | ||
| Hazard Symbols: XI, Risk Phrases: 36/37/38, Safety Phrases: 26-37/39 | ||
|
PRICES |
||
|
|
|