3-INDOLEALDEHYDE
PRODUCT IDENTIFICATION
487-89-8
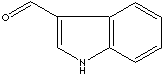
1H-Indole-3-carboxaldehyde; 3-Indolecarbaldehyde;
Indole-3-carboxaldehyde; Indole-3-aldehyde; 3-Formylindole; beta-Indolylaldehyde; Other RN: 246045-99-8
CLASSIFICATION
EXTRA NOTES
Starting material for the synthesis of higher order indoles including isoindolo[2,1-a]indoles, aplysinopsins, and 4-substituted-tetrahydrobenz[cd]indoles.
PHYSICAL AND CHEMICAL PROPERTIES
pH
log P
240 C
EXTERNAL LINKS & GENERAL DESCRIPTION
Drug Information Portal (U.S. National Library of Medicine) - Indole-3-carbaldehyde
PubChem Compound Summary - Indole-3-carbaldehyde
KEGG (Kyoto Encyclopedia of Genes and Genomes) - Indole-3-carbaldehyde
http://www.ebi.ac.uk/chebi/ - Indole-3-carbaldehyde
http://www.ncbi.nlm.nih.gov/ - Indole-3-carbaldehyde
http://www.sigmaaldrich.com/
Application:
Reactant for preparation of ·Analgesic agents ·Hypoglycemic
agents ·Tryptophan dioxygenase inhibitors pyridyl-ethenyl-indoles
as potential anticancer immunomodulators ·Antibacterial and
antifungal agents ·Antiamoebic and cytotoxic agents ·Inhibitors
of the Dengue virus protease with antiviral activity in cell-culture
·Curcumin analogues as possible anti-proliferative &
anti-inflammatory agents ·Inhibitors of Bcl-2 family proteins
·Inhibitors of the C-terminal domain of RNA Polymerase II
as antitumor agents ·Inhibitors of TNF-α and IL-6 with anti-tubercular
activity
Local:
Indole, benzopyrrole, is a yellow crystalline powder with unpleasant aroma. It
has the pyrrole ring (five-membered unsaturated ring structure composed of four
carbon atoms and one nitrogen atom) which is fused to benzene ring. There are
tautomerer called indolenine (unsubstituted 3H-indole) and structural isomer,
isoindole. But they are unstable. Indole occurs in some plants or in coal tar,
and is formed in the intestine during putrefaction and by certain cultures of
bacteria. it is commercially synthesized from phenylhydrazine and pyruvic acid. Indole structure is a
motif in nature. Prominent examples include tryptophan (aromatic side chain amino acid), serotonin
(neurotransmitter), auxin (plant growth hormone), and indigo (plant colorant).
One more interesting point is all these compounds have functional branches at
3
position.
Indole is used in perfumery and in preparing tryptophan, one of the 20 amino acids
commonly found in animal proteins. It has important application in the industry
of plant growth. It is used to prepare indoleacetic acid (auxin) and other
plant growth substances which help the development of roots in plant. It is used
to make selective herbicides. Indole and its derivatives are widely used in
making perfumes, dyes, agrochemicals and medicines.
APPEARANCE
HAZARD OVERVIEW
GHS
PICTOGRAMS
HAZARD STATEMENTS
P STATEMENTS
RISK PHRASES
SAFETY PHRASES
22-24/25