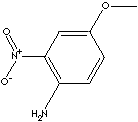
PRODUCT IDENTIFICATION
H.S.CODE
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
260 C
APPLICATIONS
- Michael addition
- Reduction
- Henry Reaction (Nitro-aldol reaction)
- Nef reaction
- O-Alkylation
- Cycloaddition
- Substitution, Elimination, Conversion reaction
- Alkylation, Acylation, and Halogenation
Anisole is one of the simplest aromatic compound to which ether group is linked. But it is different with aromatic compounds like furan where the oxygen is a part of the ring. Anisole, C6H5OCH3 (methyl phenyl ether), is a clear liquid that is soluble in ether and alcohol; insoluble in water; boiling point 155 C. Anisole and its derivatives are used as solvents and in perfumery. Anisole can be obtained from anise seed. Anisic acid, p-methoxybenzoic acid, is a part of cresol class antiseptic compounds. It is also used as an insect repellent and ovicide. Anisole, anisic acid, and their derivatives are also widely used in chemical reaction as intermediates to obtain target materials such as dyes, pharmaceuticals, perfumes, photoinitiators and agrochemicals. Anisidines, methoxyanilines, are used as intermediates for the synthesis of azo dyes, pigments and other chemical compounds.
2-Nitro-p-anisidine is used as an intermediate for the synthesis of dyes, pigments and other chemical compounds.
APPEARANCE
98.5% min