| CAS
NO. |
81-20-9 |
|
| EINECS
NO. |
201-333-6 |
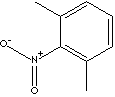
| FORMULA |
C8H9NO2 |
| MOL
WT. |
151.16 |
| H.S.
CODE |
2904.20
|
| TOXICITY |
|
| SYNONYMS |
1,3-Dimethyl-2-nitrobenzene;
2,6-Dimethylnitrobenzene;
|
|
2-Nitro-1,3-dimethylbenzene;
|
|
SMILES |
|
|
CLASSIFICATION
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES
|
| PHYSICAL
STATE |
Yellow crystals, needles or liquid
|
| MELTING POINT |
14
- 16 C |
| BOLING
POINT |
225
C |
| SPECIFIC GRAVITY |
1.112
|
| SOLUBILITY
IN WATER |
0.1g/100ml
at 19C |
| pH |
|
| VAPOR DENSITY |
5.21 |
| AUTOIGNITION
|
|
| NFPA
RATINGS |
Health:2 ; Flammability: 2; Reactivity: 0 |
REFRACTIVE
INDEX
|
|
| FLASH
POINT |
87
C
|
| STABILITY |
Stable
under ordinary conditions |
|
APPLICATIONS
|
The prefix nitro- indicates the presence of NO2- radical, while nitrate refers
to any salt or ester of nitric acid or the NO3- anion. Nitroso- is the prefix
indicating presence of the group -NO and azo- is for -N=N- group. Some range of
organic compounds containing nitrogen include nitro compounds (RNO2 ), nitroso
compounds (RNO), amines (R3N ), amino acids, and natural alkaloids or
nucleotides. The nitrogen ion in nitro compounds is trigonally planar with 120°
angles. There are two resonance bonds so that the two oxygens are equivalent.
Nitro compounds are strongly basic due to electron withdrawing both inductively
and mesomerically. Historically, they
are abundant in dyes and explosives. Nitro compounds, organic hydrocarbons having one or more NO2
groups bonded via nitrogen to the carbon framework, are versatile intermediate
in organic synthesis.
- Michael
addition
- Reduction
- Henry
Reaction (Nitro-aldol reaction)
- Nef
reaction
- O-Alkylation
- Cycloaddition
- Substitution, Elimination, Conversion
reaction
- Alkylation,
Acylation, and Halogenation
Nitro compounds are readily reduced into amines when reacted with hydrochloric
acid. Aromatic nitro compounds also yield anilines, aromatic amine compounds.
Nitro compounds are cleaved into two parts by the addition of a molecule of
water to produce carbonyl compounds (aldehydes or ketones). Aromatic nitro
compounds undergo nucleophilic substitutions to be replaced by the hydroxide
anion, resulting in the creation of phenol compounds and by alkoxy nucleophiles
to corresponding ethers.
|
| SALES
SPECIFICATION |
|
APPEARANCE
|
Yellow crystals, needles or liquid
|
| ASSAY
(HPLC) |
99.0%
min
|
| MOISTURE |
0.1%
max |
|
SOLIDIFYING POINT
|
15.3
C |
| TRANSPORTATION |
| PACKING |
200kgs
in Drum, Iso-Tank |
| HAZARD CLASS |
6.1
(Packing Group: II) |
| UN
NO. |
1665 |
| OTHER
INFORMATION |
|
Hazard
Symbols: XN, Risk Phrases: 33, Safety
Phrases: 28A-37-45 |
