PRODUCT IDENTIFICATION
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
APPLICATIONS
APPEARANCE
99.0% min
CHLORIDE
0.02% max
INSOLUBLES
0.2% max
SULFATE
0.05% max
OTHER INFORMATION
|
|
|
| POTASSIUM PERMANGANATE | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 7722-64-7 |
|
| EINECS NO. | 231-760-3 | |
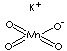
| FORMULA | KMnO4 | |
| MOL WT. | 158.03 | |
| H.S. CODE | ||
| TOXICITY |
|
|
| SYNONYMS | Permanganic acid, potassium salt; | |
| C.I. 77755; Chameleon mineral; Condy's crystals; Kaliumpermanganat; Permanganate de potassium; Permanganate of potash; Permanganato potasico; Potassio (permanganato di); Potassium (permanganate de); Potassium manganate (Ⅶ); | ||
| RAW MATERIALS |
|
|
|
CLASSIFICATION |
||
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE | dark purple crystals | |
| MELTING POINT | ||
| BOILING POINT | ||
| SPECIFIC GRAVITY | 2.7 | |
| SOLUBILITY IN WATER | Moderately soluble | |
| pH | ||
| VAPOR DENSITY | ||
|
AUTOIGNITION |
|
|
|
NFPA RATINGS |
Health: 1; Flammability: 0; Instability: 1; Special Hazard: OX | |
|
REFRACTIVE INDEX |
||
| FLASH POINT | ||
| STABILITY | Stable under ordinary conditions. Hygroscopic. | |
|
APPLICATIONS |
||
| The common oxidation states of manganese are +2, +3, +4, +6 and +7. Manganese compound such as permanganic acid in oxidation state +7 are powerful oxidizing agent when reduced to +2 and to +4 (in alkaline solution). Permanganic acid finds application as a antiseptic and a disinfectant. Permanganic acid (HMnO4) is an unstable, existing only in aqueous solution and its salts called permanganates which has a deep purple color in aqueous solution Usually manganate(Ⅶ) compounds are deep purple-colored and Mn+2 compounds are pink colored. Permanganates have the MnO4- anion and used as strong oxidizing agents. Sodium permanganate (MnNaO4) and potassium permanganate (MnKO4) are the sodium and potassium salt of permanganic acid respectively. Permanganate salts have bactericidal, fungicidal, astringent, and oxidizing properties. They are used as disinfectant in solution as a topical anti-infective. It is also used as an antidote for certain poisons (phosphorus). They are used deodorizers and to treat some parasitic diseases of fish, and used in treatment of drinking water. Diols can be produced from the oxidation of carbon-carbon solid bonds, with acidic potassium permanganate. Stronger permanganate solutions can oxidize a alkane group on an aromatic ring to a carboxyl group. Thermal decomposition of KMnO4 produce Layered MnO2 having characteristics for battery applications. Permanganate are versatile oxidants in organic chemistry including heterogeneous oxidations and diol productions. It is used as a anti-infective, germicide, bactericide, disinfectant and colorants. | ||
| SALES SPECIFICATION | ||
|
APPEARANCE |
orange to brown powder | |
| ASSAY |
99.0% min |
|
|
CHLORIDE |
0.02% max |
|
|
INSOLUBLES |
0.2% max |
|
|
SULFATE |
0.05% max |
|
| TRANSPORTATION | ||
| PACKING | 50kgs in bag | |
| HAZARD CLASS | 5.1 (Packing Group: II) | |
| UN NO. | 1490 | |
|
OTHER INFORMATION |
||
| Hazard Symbols: O T N, Risk Phrases: 43-49-50/53-9, Safety Phrases: 53-17-26-27-36/37/39-45 | ||
|
|
|