PRODUCT IDENTIFICATION
108-24-7
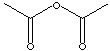
C4H6O3
102.09
H.S. CODE
CLASSIFICATION
GENERAL DESCRIPTION
Acetic Anhydride is a clear, colorless liquid with a very pungent, penetrating, vinegar-like odor that combines with water to form acetic acid. It is soluble in ether, chloroform and benzene. It reacts with alcohols Acetic anhydride is prepared commercially in either of two ways. : Acetaldehyde is converted into acetic anhydride by atmospheric oxidizing the liquid acetaldehyde in the presence of a metal acetate as the catalyst (Oxidation process). ; The ketene (or acetylene) created form hot vapor of acetic acid is converted into acetic anhydride by reacted with acetic acid (Ketene process). The acyl groups (RCO) in organic anhydrides favor wide range of organic synthesis. They react with water to give carboxylic acids, with alcohols or phenols to give esters, and with ammonia and amines to give amides. Acetic anhydride is used in the manufacture of cellulose acetate having the application as a base for magnetic tape and in the manufacture of textile fibres. Also, it is heated with salicylic acid to produce acetylsalicylic acid (aspirin). It is also used in the manufacture of pigments, dyes, cellulose and pesticides etc.
PHYSICAL AND CHEMICAL PROPERTIES
Clear liquid, Strong acetic odor
MELTING POINT
- 73C
140C
1.082
Slowly soluble (Decomposes)
3.52
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
APPLICATIONS
SALES SPECIFICATION
APPEARANCE
Clear liquid
PURITY
99.50 % max
ACETIC ACID
0.50 % max
COLOR
10 Pt-Co Scale
PERMANGANATE TIME
10 min (minutes)
ALUMINUM
1 max (mg/kg)
IRON
1 max (mg/kg)
HEAVY METALS (as Pb)
1 max (mg/kg)
NONVOLATILES
0.003 max (g/100 ml )
CHLORIDES
1 max (mg/kg)
SULFUR
1 max (mg/kg)
TRANSPORTATION
1715
GENERAL DESCRIPTION OF ANHYDRIDE
Anhydrides of inorganic acids are usually oxides of nonmetallic elements. Carbon dioxide (CO2) is the anhydride of carbonic acid, dinitrogen pentoxide (N2O5) is the anhydride of nitric acid, sodium oxide is an anhydride of sodium hydroxide, phosphorus pentoxide (P2O5) is the anhydride of phosphoric acid, and sulfur trioxide (SO3) is the anhydride of sulfuric acid. An acid anhydride forms an acid; a base anhydride forms a base. Sulfur trioxide (acid anhydride) reacts with water to form sulfuric acid (an acid product). Calcium oxide (an base anhydride) reacts with water to form calcium hydroxide (a base product).
Organic anhydrides contain the carbonyl group (CO). Organic anhydrides are formed by the condensation of original acids. Lactone, an internal cyclic monoester, is an anhydride derived from the hydroxyl and carboxyl radicals. In organic chemistry, most anhydride compounds are derived from corresponding carboxylic acids. Carboxylic anhydrides, general formula (RCO)2O, are the dehydration product of two carboxylic acid molecules. The name of carboxylic anhydride is given first from the original acid, followed by the separate word "anhydride". [CH3(CH2)2CO]2O is butanoic anhydride, CH3COOCOCH2CH3 is ethanoic propanoic anhydride (or acetic propionic anhydride). Anhydrides are more reactive than the parent acids. Anhydrides are typically not target molecules, but rather they are used as intermediates for the synthesis of other organic members such as esters and amides for the industrial applications include dyes, pharmaceuticals, pesticides, plastics, fibers, curing agents, plasticizers and many others. The reactivity of carboxylic acid derivatives are in order of acyl halides > anhydrides >> esters �� acids >> amides. Anhydrides react with alcohols to form esters; acetic anhydride [(CH3CO)2O] reacts with ethanol (C2H5OH) to form ethyl acetate (CH3COOC2H5) used as a common solvent. Anhydrides also react with ammonia and primary or secondary amines to form amides. Anhydrides react with water to form their corresponding acids.