WORTMANNIN
PRODUCT IDENTIFICATION
19545-26-7
TOXICITY
Oral Rat LD50 : 4 mg/kg
H.S. CODE
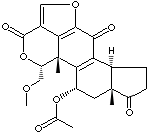
Wortmannin;
(1alpha,11alpha)-11-(Acetyloxy)-1-(methoxymethyl)-2-oxaandrosta-5,8-dieno (6,5,4-bc)furan- 3,7,17- trione;
O1C(=O)c2c3[C@]([C@H]1COC)(C1=C(C(=O)c3oc2)[C@H]2[C@] (C[C@H] 1OC(=O)C)(C(=O)CC2)C)C
CLASSIFICATION
Antibiotic, Antifungal, Hormone antagonist, Immunosuppressive, Protein Kinase Inhibitor, Serotonin antagonist
EXTRA NOTES
A phospholipase D antagonist; also inhibits PI3-kinase; inhibits alloantigen-specific T cell activation and cytotoxic T cell responses in human tumor cell lines.
PHYSICAL AND CHEMICAL PROPERTIES
Insoluble
Soluble in methanol (5 mg/ml), ethanol
REFRACTIVE INDEX
NFPA RATINGS
Health hazard: 4, Fire: 0, Reactivity Hazard: 1
AUTOIGNITION
FLASH POINT
EXTERNAL LINKS & GENERAL DESCRIPTION
Drug Information Portal (U.S. National Library of Medicine) - Wortmannin
PubChem Compound Summary - Wortmannin
Drug Bank - Wortmannin
KEGG (Kyoto Encyclopedia of Genes and Genomes) - Wortmannin
http://www.ebi.ac.uk/ - Wortmannin
http://www.ncbi.nlm.nih.gov/ - Wortmannin
http://www.molbiolcell.org/Inhibition of Endosome Fusion by Wortmannin Persists in the Presence of Activated rab5
http://pubs.rsc.org/
Recent
synthetic and biological studies of the viridin class of steroidal
furans have revealed multiple opportunities for fundamental discoveries
as well as advanced drug design. Wortmannin is a potent enzyme inhibitor
that binds to the ATP site of important regulatory kinases such
as PI-3 kinase and Polo-like kinase. The natural product shares
a unique mechanism-based biological activation pathway with other
viridins. Furthermore, while there have been several encouraging
approaches toward the total synthesis of these compounds, there
is still ample room for improvements in synthetic strategies and
tactics, and the development of structurally simplified analogs
that exert more specific biological effects and are devoid of toxicity
issues that have thwarted the clinical development of the parent
compounds.
http://www.sigmaaldrich.com/
Biochem/physiol
Actions:Wortmannin is a potent and specific phosphatidylinositol
3-kinase (PI3-K) inhibitor with an IC50 of 2-4 nM. Inhibition of
PI3-K/Akt signal transduction cascade enhances the apoptotic effects
of radiation or serum withdrawal and blocks the antiapoptotic effect
of cytokines. Inhibition of PI3-K by wortmannin also blocks many
of the short-term metabolic effects induced by insulin receptor
activation.
Local:
APPEARANCE
ASSAY
98.0% min
3462
HAZARD OVERVIEW
OSHA Hazards:Delayed target organ effects, Highly toxic by inhalation, Highly toxic by ingestion, Highly toxic by skin absorption. Target Organs: Hear
GHS
Danger
PICTOGRAMS

HAZARD STATEMENTS
H300-H310-H330
P STATEMENTS
P260-P264-P280-P284-P302 + P350-P310
![]()
RISK PHRASES
26/27/28
SAFETY PHRASES
28-36/37-45