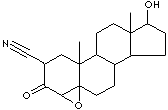
PRODUCT IDENTIFICATION
H.S. CODE
TOXICITY
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
Stable under ordinary conditions
EXTERNAL LINKS & GENERAL DESCRIPTION
- This is used to control the symptoms of adrenal cortical hyperfunction in Cushings and primary aldosteronism. However, it appears to be less effective than metyrapone for Cushing's syndrome.
- Trilostane offers an alternative endocrine treatment in advanced postmenopausal breast cancer. It has a unique mode of action; it is an allosteric modulator of the oestrogen receptor and targets both the oestrogen- and growth factor-dependent pathways through which oestradiol stimulates cell proliferation. In clinical trials, trilostane has been shown to be an effective treatment for breast cancer in patients who have relapsed after receiving treatment with one or more forms of endocrine therapy.6 It may also have a role in the treatment of prostate cancer.
- It is used to treat resistant oedema due to increased aldosterone secretion in cirrhosis, nephrotic syndrome, and congestive heart failure (with glucocorticoid replacement therapy). (http://www.patient.co.uk/)
Canine hyperadrenocorticism (HAC) has been most commonly treated with the adrenolytic drug o, p'-DDD (mitotane). However, it is well recognised that mitotane has several side effects, is associated with a high frequency of relapses and is not without risk to owners. Other drugs such as ketoconazole and l-deprenyl (selegeline) have also been investigated for the treatment of HAC. Ketoconazole, whilst effective, is hepatotoxic. L-deprenyl, whilst safe, has limited efficacy in reducing circulating cortisol concentrations. Recently it has been suggested that trilostane was an effective treatment for canine hyperadrenocorticism. Several workers in the field have subsequently used trilostane in canine pituitary dependent and adrenal dependent hyperadrenocorticism, in feline hyperadrenocorticism and in equine Cushing's syndrome. Trilostane is a synthetic, orally active steroid analogue. It can act as a competitive inhibitor of the 3ß hydroxysteroid dehydrogenase enzyme system and thereby inhibit the synthesis of several steroids, including cortisol and aldosterone. This blockade is reversible and seems to be dose-related. (http://www.fincaverde.de/)
Pharmacological Actions- Steroidal Abortifacient
- Adrenocortical suppressant
- Anthelmintic
- Antineoplastic
- Enzyme inhibitor
- Hormone
|
|
APPEARANCE
99.0% min (HPLC)
HEAVY METALS
20ppm max
IMPURITY
1.0% max (Total), 0.5% max (Individual)
LOSS ON DRYING
0.5% max