PRODUCT IDENTIFICATION
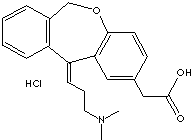
140462-76-6 (hydrochloride)
TOXICITY
H.S. CODE
CLASSIFICATION
EXTRA NOTES
Olopatadine is a histamine H1 receptor antagonist and mast cell stabilizer. The low level of occupancy H1 receptors in the brain explains the low sedation effect of olopatidine. It is believed that olopatidine is a substrate for P-glycoprotein, which limits its brain penetration.
PHYSICAL AND CHEMICAL PROPERTIES
white to off-white crystalline powder
Sparingly soluble
Soluble methanol
REFRACTIVE INDEX
NFPA RATINGS
Health hazard: 2, Fire: 0, Reactivity Hazard: 0
AUTOIGNITION
FLASH POINT
EXTERNAL LINKS & GENERAL DESCRIPTION
Drug Information Portal (U.S. National Library of Medicine) - Olopatadine
PubChem Compound Summary - Olopatadine
http://www.drugbank.ca/
Olopatadine is a selective histamine H1 antagonist that binds to the histamine
H1 receptor. This blocks the action of endogenous histamine, which subsequently
leads to temporary relief of the negative symptoms brought on by histamine.
Olopatadine is devoid of effects on alpha-adrenergic, dopamine and muscarinic
type 1 and 2 receptors.
http://dmd.aspetjournals.org/
Effects
of Olopatadine, a New Antiallergic Agent, on Human Liver Microsomal
Cytochrome P450 Activities - Olopatadine, a new histamine H1 receptor-selective antagonist, is a
tricyclic drug containing an alkylamino moiety. Some compounds containing a
similar alkylamino group form a cytochrome P450 (P450) -iron (II)-nitrosoalkane
metabolite complex [metabolic intermediate complex (MIC)], thereby causing
quasi-irreversible inhibition of the P450. There was concern that olopatadine
might also form MICs, therefore, the present investigation was undertaken to
explore this possibility. We identified the enzymes catalyzing olopatadine
metabolism and investigated the effect of olopatadine on human P450 activities.
During incubation with human liver microsomes in the presence of a
NADPH-generating system, olopatadine was metabolized to two metabolites, M1
(N-monodemethylolopatadine) and M3 (olopatadine N-oxide) at
rates of 0.330 and 2.50 pmol/min/mg protein, respectively. Troleandomycin and
ketoconazole, which are both selective inhibitors of CYP3A, significantly
reduced M1 formation but specific inhibitors of other P450 isozymes did not
decrease M1 formation. Incubation of olopatadine with cDNA-expressed human P450
isozymes confirmed that M1 formation was almost exclusively catalyzed by CYP3A4.
The formation of M3 was enhanced byN-octylamine and was inhibited by
thiourea. High specific activity of M3 formation was exhibited by cDNA-expressed
flavin-containing monooxygenase (FMO)1 and FMO3. Olopatadine did not inhibit
P450 activities when it was simultaneously incubated with substrates for
different P450 isozymes. Also, P450 activities in human liver microsomes were
unaffected by pretreatment with olopatadine or M1. Furthermore, spectral
analysis revealed that neither olopatadine nor M1 formed an MIC. Therefore, it
is unlikely that olopatadine will cause drug-drug interactions involving P450
isozymes.
http://jnm.snmjournals.org/
Cerebral
Histamine H1 Receptor Binding Potential Measured with PET Under
a Test Dose of Olopatadine, an Antihistamine, Is Reduced After Repeated
Administration of Olopatadine
APPEARANCE
white to off-white crystalline powder
IDENTIFICATION
passes Test
PURITY
98.5% min
SOLUTION CLARITY
0.1 Au max
RELATED SUBSTANCES
E-Isomer 0.5% max, Individual Impurity 0.05% max, Sum Impurity 1.0% max
LOSS ON DRYING
0.5% max
HEAVY METALS
10ppm max
RESIDUE ON IGNITION
0.1% max
6.1 (Packing group: III)
GHS
OSHA Hazards: Toxic by ingestion
Danger
PICTOGRAMS


HAZARD STATEMENTS
H301
Toxic if swallowed.
H400 Very toxic to aquatic
life.
PRECAUTIONARY STATEMENTS
P273 Avoid release to the environment.
P301
+ P310 IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician.
![]() T
Toxic
T
Toxic![]() N
Dangerous for the environment
N
Dangerous for the environment
RISK PHRASES
25
Toxic if swallowed
50
Very Toxic to aquatic organisms
SAFETY PHRASES
45
In case of accident or if you feel unwell, seek medical
advice immediately (show label where possible)
61 Avoid release to the environment. Refer to special
instructions safety data sheet