PRODUCT IDENTIFICATION
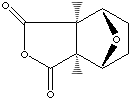
200-263-3
H.S. CODE
TOXICITY
Oral-Human LDLO - 0.428 mg/kg
CLASSIFICATION
Acid anhydride, Monoterpene, Phosphatase inhibitor
EXTRA NOTES
Inhibitor of protein phosphatase 2A.
A toxic compound, isolated from the Spanish fly or blistering beetle (Lytta (Cantharis) vesicatoria) and other insects. It is a potent and specific inhibitor of protein phosphatases 1 (PP1) and 2A (PP2A). This compound can produce severe skin inflammation, and is extremely toxic if ingested orally.
PHYSICAL AND CHEMICAL PROPERTIES
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
Stable under ordinary conditions
EXTERNAL LINKS & GENERAL DESCRIPTION
Wikipedia Linking - Cantharidin
Drug Information Portal (U.S. National Library of Medicine) - Cantharidine
PubChem Compound Summary - Cantharidine
http://www.ebi.ac.uk/chebi/ - Cantharidin
KEGG (Kyoto Encyclopedia of Genes and Genomes) - Cantharidin
http://www.faidherbe.org/
The cantharidin was first isolated by Robiquet, a French chemist in 1810. It has
an important role in the ecology of different kinds of insects that use or
produce it as a defense ability to preserve their eggs from predators. In the ancient world, the dried spanish flies had
the reputation of aphrodisiac virtues. In
fact, these supposed properties are not attested neither in theory nor in
experimentation. The cantharidin seems to be a dangerous substance with the same
toxicity as the most violent poisonus like strychnin.
Journal
of Chinese Pharmaceutical Sciences
Improved synthesis and
structure identification of L-histidine norcantharimide, a potent
PP2A inhibitor was reported. Condensation between norcantharidin
and L-histidine in 95% EtOH at reflux temperature affords L-histidine
norcantharimide in 97.0% yield which is much higher compared with
literature, and more importantly, the configuration is retained.
The chemical structure of the compound was re-elucidated through
IR, FAB-MS, 1H NMR, 13C NMR and 2D NMR (1H, 13C-COSY and HMBC),
the fundamental physical data, including optical data being also
firstly reported. Preliminary cytotoxicity evaluation showed that
the target compound was probably more potent than norcantharidin
against a panel of human cancer cell lines. Design and synthesis
of amino acid (nor) cantharimides would provide a convenient and
rational structure modification of (nor) cantharidin and open new
avenues to explore new promising candidates.
APPEARANCE
ASSAY
98.0% min (GC)
Hazard Overview
OSHA Hazards: Target Organ Effect, Highly toxic by ingestion, Irritant. Target Organs: Kidney, Cardiovascular system.
GHS
PICTOGRAMS

HAZARD STATEMENTS
H300 Fatal if swallowed.
H315 Causes skin irritation.
H319
Causes serious eye irritation.
H335 May cause respiratory irritation.
PRECAUTIONARY STATEMENTS
P261 Avoid breathing dust/ fume/ gas/ mist/ vapours/ spray.
P264
Wash hands thoroughly after handling.
P301 + P310 IF SWALLOWED:
Immediately call a POISON CENTER or doctor/ physician.
P305 +
P351 + P338 IF IN EYES: Rinse cautiously with water for several
minutes. Remove contact lenses, if present and easy to do. Continue
rinsing.
![]() T+
Very Toxic
T+
Very Toxic
RISK PHRASES
28 Very Toxic if swallowed
36/37/38 Irritating to eyes, respiratory system and skin
SAFETY PHRASES
45 In case of accident or if you feel unwell, seek medical advice immediately (show
label where possible)
53 Avoid exposure - obtain special instruction before use