|
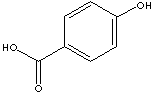
p-HYDROXYBENZOIC ACID
| ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 99-96-7 |
|
| EINECS NO. | 202-804-9 | |
| FORMULA | HOC6H4COOH | |
| MOL WT. | 138.12 | |
|
H.S. CODE |
2918.29.2200 | |
|
TOXICITY |
Oral rat LD50: > 10 gm/kg | |
| SYNONYMS | 4-Hydroxybenzoic Acid; p-Salicylic Acid; p-HBA; | |
| 4-Hydroxybenzenecarboxylic acid; Acido p-idrossibenzoico (Italian); 4-Hydroxybenzoova Kyselina; 4-Carboxyphenol; 4-Hydroxybenzoesäure (German); ácido 4-hidroxibenzoico (Spanish); Acide 4-hydroxybenzoïque (French); 4-Carboxyphenol; Acido p-idrossibenzoico; p-Oxybenzoesaure; | ||
| SMILES | c1(C(O)=O)ccc(O)cc1 | |
|
CLASSIFICATION |
Carboxylic Acid, Preservative |
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE | white crystalline powder | |
| MELTING POINT | 215 - 217 C | |
| BOILING POINT | ||
| SPECIFIC GRAVITY |
1.48 | |
| SOLUBILITY IN WATER | slightly soluble (5000 mg/l at 25 C) | |
| SOLVENT SOLUBILITY |
soluble in hot alcohol, ether slightly soluble in chloroform practically insoluble in carbon disulfide | |
| VAPOR DENSITY |
| |
| pKa | 4.54 (Dissociation Constant at 20 C) | |
| log Pow | 1.58 (Octanol-water) | |
| VAPOR PRESSURE | (mmHg at 25 C) | |
| HENRY'S LAW | 1.13E-11 (atm-m3/mole at 25 C) | |
| OH RATE | 1.30E-11 (cm3/molecule-sec at 25 C Atmospheric) | |
| AUTOIGNITION |
| |
| NFPA RATINGS | Health: 1; Flammability: 0; Reactivity: 0 | |
| REFRACTIVE INDEX |
||
| FLASH POINT |
| |
| STABILITY |
Stable under ordinary conditions | |
|
GENERAL DESCRIPTION & EXTERNAL LINKS |
||
|
p-Hydroxybenzoic Acid, one of three crystalline derivatives of benzoic acid, is
a white crystalline powder; slightly soluble in water, soluble in hot alcohol,
ether; melts at 215 C. It contains both hydroxyl and carboxyl group at
para-position, which react with either acid or alcohol. p-Hydroxybenzoic acid
is used for the preperaion of biocides, antiseptics and bacteriostatic agents.
It is used as a chemical intermediate for synthetic drugs, pharamceuticals, dyes
and plasticizers. Wikipedia Linking: http://en.wikipedia.org/wiki/P-hydroxybenzoic_acid http://onlinelibrary.wiley.com/ http://www.articlesbase.com/ |
||
| SALES SPECIFICATION | ||
|
APPEARANCE |
white crystalline powder | |
|
ASSAY |
99.0% min |
|
|
SOLUBILITY |
Clear and transparent |
|
|
LOSS ON DRYING |
0.5% max |
|
|
ASH |
0.15% max |
|
|
SULFATE |
0.05% max |
|
|
CHLORIDE |
0.02% max |
|
|
PHENOL |
0.1% max |
|
|
SALICYLIC ACID |
0.1% max |
|
|
MELTING POINT |
213 - 216 C | |
| TRANSPORTATION | ||
| PACKING | 25kgs
in bag | |
| HAZARD CLASS | Not regulated | |
| UN NO. | ||
| OTHER INFORMATION | ||
|
Hazard
Symbols: XI, Risk Phrases: 36/37/38, Safety Phrases: 26-36
|
||