PRODUCT IDENTIFICATION
203-321-6
H.S. CODE
TOXICITY
CLASSIFICATION
RXTRA NOTES
PHYSICAL AND CHEMICAL PROPERTIES
210 - 212 C
0.97
REFRACTIVE INDEX
NFPA RATINGS
AUTOIGNITION
94 C
Stable under ordinary conditions.
EXTERNAL LINKS & GENERAL DESCRIPTION
PubChem Compound Summary - 2,4-Dimethylphenol
Local:
Cresols are methyl substituted phenols at relative to the hydroxyl group,
ortho-,
meta-, and para-cresol. There are three structural isomers. The names of the
three compounds indicate which of the hydrogens on the benzene ring portion of
the molecule have been replaced. They are obtained from coal tar or petroleum
as by-products in the fractional distillation and in coal
gasification. They are also formed as by-products during
the combustion of wood. The various isomers can
be manufactured by the methylation of phenol, toluene sulfonation
and alkaline hydrolysis, or the hydrolysis of 2-isopropyltoluene
or alkaline chlorotoluene.
Because the boiling points of these three compounds are nearly the same, a
separation of a mixture of the three into its pure components is impractical.
They are highly flammable and soluble in water, ethanol, ether, acetone and
alkali hydroxides. The mixture of cresols obtained from coal tar is called
cresylic acid, an important technical product used as a disinfectant and in the
manufacture of resins and tricresyl phosphate. Cresylic acid also refs to the
mixture of phenols containing varying amounts of xylenols, cresols, and other
high-boiling fractions, but not more than 5 percent phenol. Commercial cresols
are prepared in a wide range of grades and purities to meet the user's
requirements. It is a liquid from clear to brown and is toxic to animals
including human. It is corrosive and is a more powerful disinfectant and
antiseptic than phenol. The primary use is for sterilizing as disinfectants and
deodorizers, and pesticides. Its solution is used as household cleaners as a
disinfectant. Creosote products are
mixtures of many aromatic hydrocarbons including phenols and cresols. Creosote
obtained from coal tar is poisonous and provides protection against fungi,
shipworms, termites, and psoriasis. It is used chiefly as a wood preservative,
e.g., in wooden poles, railroad ties, and timber. They are also used as animal
and bird repellents. Animals may suffer skin irritation or ulceration from
creosote treated wood. Coal tar creosote and its derivatives are the most widely
used wood preservatives. Wood tar creosote is a mixture of chiefly guaiacol,
creosols and other phenolic compounds obtained from wood tar (mainly beech) by
distillation between 203 and 220 C. It is insoluble in water, soluble in
methanol, acetone. It is used as an external antiseptic, expectorant, gastric
sedative, deodorant, and as an antiseptic parasiticide veterinary use in the
form of creosote carbonate. It is used in the synthesis of pharmaceuticals and
vanillin. Each cresols are used as
solvents or disinfectants and as useful as raw materials for various chemical
products including;
- Antiseptics, disinfectants
- Fragrances, deodorizing, odor-enhancer
- Resins (phenol-formaldehyde, phenolic, and epoxy) and their additives
- Phosphate esters (plasticizers)
- Herbicides and pharmaceuticals
- Rubber and plastic antioxidants
- Dyes and pigments
- Household cleaners and automotive degreasers
- Solvent and paints
- Lubricating oils, gasoline additives
- Adhesives
- Fiber and wood preservatives
- UV- absorbers and photographic chemicals
- Ore flotation agents
Cresols undergo electrophilic substitution reactions such as chlorination, bromination, sulfonation and nitration at the vacant position. They also undergo condensation reactions with aldehydes, ketones or dienes. O-cresol is a starting material for the synthesis of herbicides such as 4,6-dinitro-o-cresol (DNOC) and 2-methyl-4-chlorophenoxyacetic acid (MCPA). Meta-cresol is used in the manufacture of explosives. Meta and para-cresol are used in phenol-formaldehyde resins and are converted to tricresyl phosphate used as a plasticizer and gasoline additive and antioxidants such as di-tert-butylcresols (BHT). Ortho- and para-cresols are used in the production of lubricating oils and motor fuels.
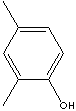
Xylenols, dimethylphenol, are isomeric substituted phenols with two methyl substituent at either the ortho-, meta- or para position relative to the hydroxyl group. They have similar characteristics and applications with cresols.
Isomers:
|
Xylenol |
CAS RN |
M.P C |
B.P C |
| 2,3-Xylenol | 526-75-0 | 70-73 | 217 |
| 2,4-Xylenol | 105-67-9 | 25-26 | 211-212 |
| 2,5-Xylenol | 95-87-4 | 63 - 65 | 212 |
|
2,6-Xylenol |
576-26-1 |
43-45 |
203 |
| 3,4-Xylenol | 95-65-8 | 62 - 68 | 227 |
| 3,5-Xylenol | 108-68-9 | 64 | 219 |
APPEARANCE
ASSAY (G.C)
99.0% min
MOISTURE (K.F.)
0.1% max
ISOMER IMPURITY
0.5% max
HAZARD OVERVIEW
Toxic by ingestion, Toxic by skin absorption, Corrosive
GHS
PICTOGRAMS



HAZARD STATEMENTS
H311 Toxic in contact with skin
H314 Causes severe
skin burns and eye damage
H318 Causes serious eye damage
H411
Toxic to aquatic life with long lasting effects
PRECAUTIONARY STATEMENTS
P273 Avoid release
to the environment
P280 Wear protective gloves/protective clothing/eye protection/face
protection
P301 + P330 + P331 IF SWALLOWED:
rinse mouth. Do NOT induce vomiting
P302 + P350: IF ON SKIN: Gently wash
with plenty of soap and water
P305 + P351 + P338 IF IN EYES: Rinse cautiously
with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing
P310 Immediately call
a POISON CENTER or doctor/physician
P312 Call a POISON CENTER or doctor/physician
if you feel unwell
RISK PHRASES
24/25 Toxic in contact with skin and if swallowed
34
Causes burns
51/53 Toxic to aquatic organisms, may
cause long-term adverse effects in the aquatic environment.
SAFETY PHRASES
24 Avoid contact with skin
26 In case of contact
with eyes, rinse immediately with plenty of water and seek medical
advice
37 Wear suitable gloves.