PRODUCT IDENTIFICATION
H.S. CODE
TOXICITY
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
AUTOIGNITION
640 C
NFPA RATINGS
REFRACTIVE INDEX
APPLICATIONS
APPEARANCE
ASSAY
25kgs in bag
|
|
|
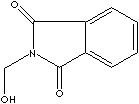
| N-HYDROXYMETHYL PHTHALIMIDE | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 118-29-6 |
|
| EINECS NO. | 204-241-4 | |
| FORMULA | C9H7NO3 | |
| MOL WT. | 177.16 | |
|
H.S. CODE |
2925.19 | |
|
TOXICITY |
||
| SYNONYMS | n-(Hydroxymethyl)-Phthalimide; | |
| 2-(Hydroxymethyl)-1H-Isoindole-1,3(2H)-dione; Hydroxymethylphthalimide; N-Methylolphthalimide; Oxymethylphthalimide; Phthalimidomethyl alcohol; | ||
| SMILES |
|
|
|
CLASSIFICATION |
||
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE | white powder | |
| MELTING POINT | 142 - 145 C | |
| BOILING POINT | ||
| SPECIFIC GRAVITY | ||
| SOLUBILITY IN WATER | ||
| pH | ||
| VAPOR DENSITY |
| |
|
AUTOIGNITION |
640 C | |
|
NFPA RATINGS |
||
|
REFRACTIVE INDEX |
||
| FLASH POINT | 158 C | |
| STABILITY | Stable under ordinary conditions. | |
|
APPLICATIONS |
||
| Imide refers to any compound which contains the divalent radical "-C(=O)NHC(=O)-". Imide compounds are derived from ammonia or primary amine, where two hydrogen atoms are replaced by a bivalent acid group or two monovalent acid groups, resulting in consisting of two carboxylic acid groups (or one dicarboxylic acid). In other description, Imide is a compound derived from an acid anhydride by replacing the oxygen with the =NH group. Imides are monomers to prepare polyimides that contain repeating imide groups. Aromatic polyimides have better resistance to high temperatures and corrosion than linear polyimides. Frequently, the term of imide refers to the combined forms such as maleimides, phthalimides, and succinimides which are used as plastic modifiers to improve heat-resistant, antioxidant and antifoulant properties. They are used as intermediates for the synthesis of cross-linking agents, pesticides, dyes, antiseptics and crystalline adducting agents. They are also useful compounds in the synthesis of primary amines and amino acids for the application in the field of medicine and biological research. Phthalimide, derived from phthalic anhydride with ammonium hydroxide by heating, is used in the synthesis of primary amines and amino acids. It is used to make synthetic indigo and phthalocyanine pigments which have macrocyclic structure showing striking coloring features like porphyrins (biopigments). Phthalimide has isoindole moiety. Indole structure is a motif in nature. Prominent examples include tryptophan (aromatic side chain amino acid), serotonin (neurotransmitter), auxin (plant growth hormone), and indigo (plant colorant). The radical "=NH" is called imido group. Imido is a prefix used to denote the presence in a compound of the bivalent group "=NH" attached to only acid radicals. Imine is a compound containing the bivalent "=NH" group combined with a bivalent nonacid group, as "R-HC=NH". It is produced by the condensation reactions of aldehydes or ketones with ammonia (or amines). Imino is a prefix denoting the presence of the bivalent group "=NH" attached to nonacid radicals. N-alkylated phthalimides, made from potassium phthalimide, are used for the synthesis of primary amines (Gabriel synthesis) by the hydrolysis reaction. Phthalimides are used for the preparing of synthetic indigo, pesticides, pigments, dyes, pharmaceuticals, and fungicides. N-Hydroxymethyl Phthalimide is used as an intermediate in medicine, pharmacy, dyes and pesticides. | ||
| SALES SPECIFICATION | ||
|
APPEARANCE |
white powder | |
|
ASSAY |
98.0% min | |
| TRANSPORTATION | ||
| PACKING | 25kgs in bag | |
| HAZARD CLASS | not regulated | |
| UN NO. | ||
| OTHER INFORMATION | ||
| Hazard Symbols: n/a, Risk Phrases: n/a, Safety Phrases: 24/25 | ||
|
|
|